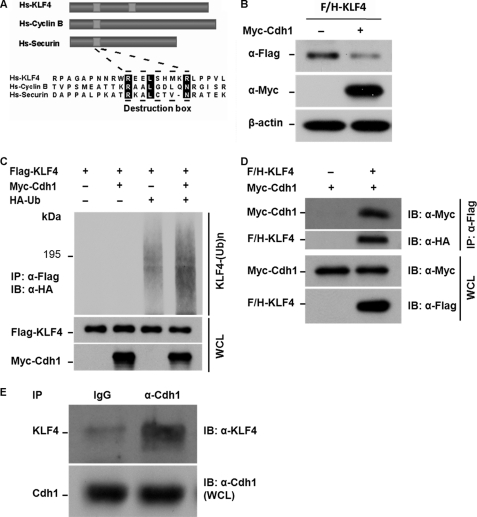
FIGURE 3.
Cdh1/APC is a putative E3 ligase that facilitates TGF-β-induced KLF4 ubiquitylation. A, identification of destruction motifs on KLF4. Like most APC substrates, KLF4 bears a conserved destruction box as indicated by sequence alignment with Cyclin B and securin. The alignment was performed using the CLUSTAL W methods. Hs, human. B, elevation of Cdh1 results in degradation of KLF4. HEK293T cells were co-transfected with FLAG- and HA-tagged KLF4 (F/H-KLF4) and Myc-tagged Cdh1 (Myc-Cdh1). Cells were collected 40 h after the transfection. Expression levels of F/H-KLF4 and Myc-Cdh1 were measured by immunoblotting (IB) using the antibodies against FLAG and the Myc tag, respectively. β-Actin was used as loading control. KLF4 protein levels were dropped in cells with expression of Cdh1. C, overexpression of Cdh1 enhances KLF4 ubiquitylation. FLAG-tagged KLF4 (Flag-KLF4) was transfected into HEK293T cells together with Myc-Cdh1 and HA-tagged ubiquitin (HA-Ub). Transfected cells were treated with MG132 for 6 h. The accumulated ubiquitin-conjugated FLAG-KLF4 was examined by immunoprecipitation (IP) using anti-FLAG M2 affinity gel. KLF4 ubiquitin conjugates were then detected by immunoblotting using antibody against HA. The expression of FLAG-KLF4 and Myc-Cdh1 in the whole cell lysates (WCL) was estimated by immunoblotting. Overexpression of Cdh1 significantly enhances the formation of KLF4 ubiquitin conjugates. D, KLF4 interacts with Cdh1 in vivo. F/H-KLF4 and Myc-Cdh1 were co-transfected into HEK293T cells. Interaction between KLF4 and Cdh1 was measured by immunoprecipitation using anti-FLAG-M2 gel (Flag-KLF4) following by immunoblotting using anti-Myc (Myc-Cdh1). Expression of both FLAG-KLF4 and Myc-Cdh1 was estimated by immunoblotting using whole cell lysates. E, endogenous KLF4 interacts with Cdh1 in Mv1Lu cells. Mv1Lu cells were treated by TGF-β and applied to immunoprecipitation assay using anti-Cdh1 antibody and subsequent immunoblotting using anti-KLF4 antibody. Cdh1 was pulled down with KLF4 immunoprecipitation. The expression of Cdh1 in the whole cell lysates was measured by immunoblotting.