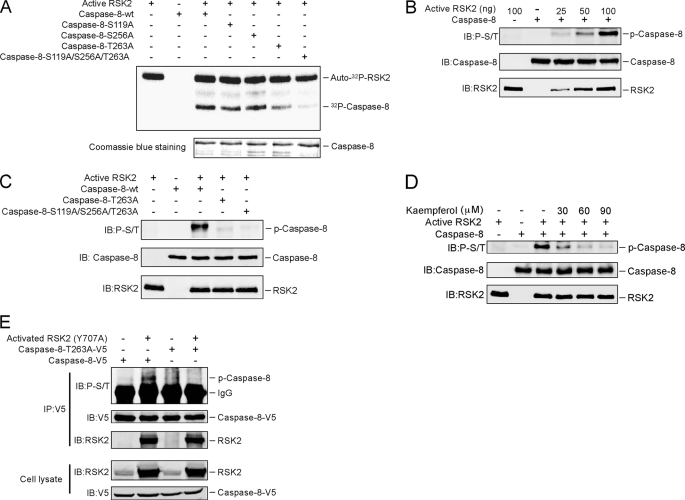
FIGURE 2.
RSK2 phosphorylates caspase-8 (Thr-263). A, Thr-263 is a major phosphorylation site on caspase-8 for RSK2 in vitro. The indicated caspase-8 mutant proteins were purified and subjected to an in vitro kinase assay with 100 ng of active RSK2, 10 μCi of [γ-32P]ATP, and 50 μm unlabeled ATP. The 32P-labeled bands were visualized by autoradiography. B, the phospho-Ser/Thr (p-S/T) antibody recognizes the phosphorylation of caspase-8 in vitro. An in vitro kinase assay was performed with the caspase-8 protein together with increasing amounts of active RSK2. Phosphorylation of caspase-8 was detected by Western blot with a phospho-Ser/Thr antibody. C, confirmation of caspase-8 phosphorylation sites by Western blot. An in vitro kinase assay was performed with caspase-8-WT or caspase-8 mutants together with 100 ng of active RSK2 and 200 μm unlabeled ATP. The phosphorylated caspase-8 (P-Caspase-8) was visualized by Western blot with a phospho-Ser/Thr antibody. D, kaempferol inhibits RSK2-mediated caspase-8 phosphorylation in vitro. An in vitro kinase assay was conducted with 100 ng of active RSK2, 5 μg of caspase-8, 200 μm unlabeled ATP, and the indicated dose of kaempferol. Phosphorylated caspase-8 was visualized by Western blot with a phospho-Ser/Thr antibody. E, constitutively active RSK2 phosphorylates caspase-8 ex vivo. HEK293 cells were transfected with caspase-8-WT or the caspase-8-T263A mutant with or without constitutively active RSK2 (Y707A). Cells were cultured for 30 h, and then proteins were extracted. The caspase-8 proteins were immunoprecipitated (IP) with anti-V5, and phosphorylated caspase-8 was visualized by Western blot with a phospho-Ser/Thr antibody. IB, immunoblot.