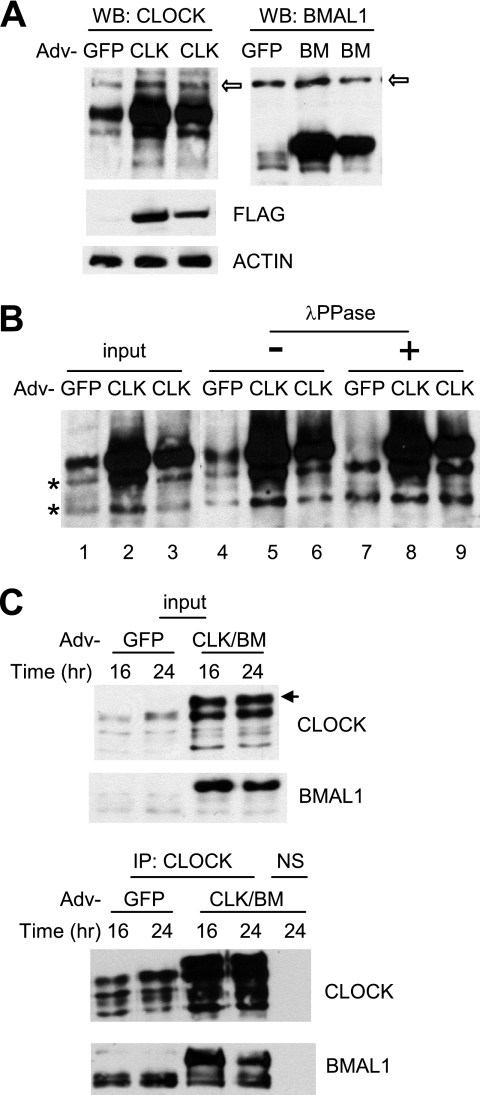
FIGURE 2.
Adenoviral CLOCK and BMAL1 are functional in cultured cells. A, adenoviral constructs overexpress exogenous CLOCK and BMAL1 proteins in MEFs. MEFs were infected with GFP, CLOCK (CLK), or BMAL1 (BM) adenovirus (Adv) and harvested 24 h later. 1× (center lane) and 0.5× (last lane of each blot) titers were used for CLOCK and BMAL1. The white arrows indicate nonspecific bands. Note that exogenous CLOCK co-migrated with a hyperphosphorylated endogenous CLOCK isoform (see B). Expression of FLAG-CLOCK was confirmed by FLAG immunoblot (center panel). B, resolution of exogenous versus endogenous CLOCK protein. MEFs were infected with GFP or CLOCK adenovirus and harvested as described above. MEF extracts (input) were immunoprecipitated and treated without (−) or with (+) λ-protein phosphatase (λPPase). Note that phosphatase treatment resulted in two non-phosphorylated CLOCK isoforms in GFP MEFs (indicated by two asterisks) but three isoforms in CLOCK MEFs, indicating that the top band is exogenous CLOCK. C, exogenous CLOCK and BMAL1 form a heterodimer. MEFs were infected with GFP or CLOCK/BMAL1 adenovirus and harvested at 16 and 24 h. Bottom panels are immunoprecipitated (IP) samples from the top panels, which are input samples for immunoprecipitation. Note an extra band in CLOCK/BMAL1 (CLK/BM) MEFs in the top panel. This is a hyperphosphorylated exogenous CLOCK isoform that can be seen only when co-infected with BMAL1 adenovirus. NS, nonspecific immunoprecipitation.