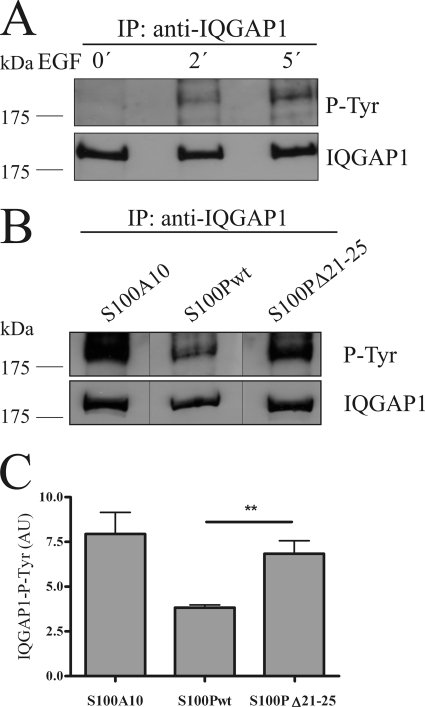
FIGURE 6.
S100P interferes with EGF-induced tyrosine phosphorylation of IQGAP1. A, HeLa cells were serum-starved and either kept unstimulated or were stimulated with 50 ng/ml EGF for 2 or 5 min. Cells were then lysed, and the cleared cell lysates were subjected to immunoprecipitation (IP) using a polyclonal anti-IQGAP1 antibody. Time-dependent tyrosine phosphorylation of the precipitated IQGAP1 was analyzed by immunoblotting using a monoclonal anti-phosphotyrosine (P-Tyr) antibody (upper panel). Reprobing of the blot with a monoclonal anti-IQGAP1 antibody was carried out to verify that equal amounts of protein were precipitated (lower panel). B, HeLa cells expressing GFP-S100Pwt, GFP-S100PΔ21–25, or YFP-S100A10 (an S100 protein incapable of binding IQGAP1 used as control) were serum-starved and stimulated with 50 ng/ml EGF for 5 min. The tyrosine phosphorylation status of the precipitated IQGAP1 was analyzed as in A. Black lines indicate that the gel was cut between the lanes. C, statistical evaluation of EGF-induced IQGAP1 tyrosine phosphorylation in the presence of different S100 derivatives. ECL signal intensities of precipitated tyrosine-phosphorylated IQGAP1 protein bands from five independent experiments (a typical example is shown in B) were quantified by densitometry, and results were corrected for the amount of IQGAP1 in the corresponding sample, as revealed by blotting for total IQGAP1. Data represent means ±S.E. Statistical significance was calculated using Student's t test (**, p < 0.01). AU, arbitrary units.