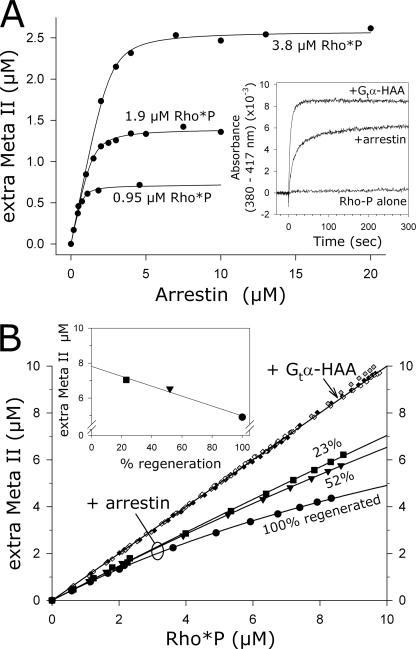
FIGURE 6.
Each arrestin molecule stabilized one receptor molecule as Meta II. A, unlabeled arrestin was titrated against three different concentrations of RhoP (5, 10, or 20 μm), and the amount of stabilized Meta II following an activating flash (19%) was measured as shown in the inset. The concentrations of Rho*P in each titration are indicated. The fitted curves reported that 0.7 arrestins bound for every light-activated rhodopsin (Rho*P). Likewise, ∼70% of Rho*P was stabilized as Meta II. Inset, binding to and stabilization of Meta II over its precursor Meta I resulted in an increase in absorbance (380–417 nm). Meta II stabilization by arrestin was quantified by comparing absorption signals to those obtained with the peptide Gtα-HAA, which stabilized all Rho*P as Meta II. Samples contained 10 μm RhoP plus 300 μm Gtα-HAA or 10 μm arrestin and were activated by a flash at t = 0 s. B, fully or partially regenerated ROS membranes containing 10 μm RhoP were sequentially photoactivated. In the presence of Gtα-HAA (300 μm), every Rho*P was stabilized as Meta II (closed diamonds, 100% regenerated ROS-P; gray diamonds, 52% regenerated ROS-P; open diamonds, 23% regenerated ROS-P). In the presence of arrestin (20 μm), different proportions of Rho*P were stabilized as Meta II, depending on the photoactivation density (circles, 100% regenerated ROS-P; triangles, 52% regenerated ROS-P; squares, 23% regenerated ROS-P). An extrapolation of the data points to full photoactivation indicated a linear relationship between the amount of stabilized Meta II and the photoactivation density (inset).