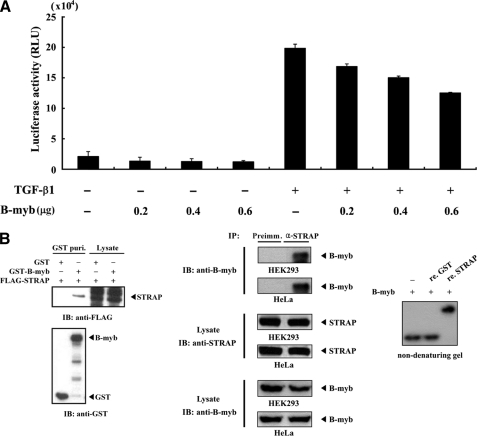
FIGURE 1.
Physical interaction of B-MYB with STRAP. A, inhibition of TGF-β-induced transcription by B-MYB. HepG2 cells were transfected with 0.3 μg of p3TP-Lux reporter and increasing amounts of B-MYB, as indicated, in the presence or absence of TGF-β1 (100 pm). B, in vivo and in vitro association of B-MYB with STRAP. GST alone and GST-B-MYB were cotransfected with FLAG-STRAP into HEK293 cells. GST fusion proteins were purified on glutathione-Sepharose beads (GST Puri.), and the amounts of complex formation and FLAG-STRAP used for the in vivo binding assay were determined by anti-FLAG antibody immunoblot (left, upper panel). Cell lysates from HEK293 or HeLa cells were subjected to immunoprecipitation using either rabbit preimmune serum (Preimm.) or anti-STRAP antibody (α-STRAP), followed by immunoblot analysis using an anti-B-MYB antibody to determine the complex formation between endogenous B-MYB and STRAP (middle). For native PAGE of the B-MYB·STRAP complex, in vitro translated 35S-labeled B-MYB was prepared with the TnT reticulocyte lysate system. 35S-Labeled B-MYB was incubated with unlabeled recombinant GST, as a control, or STRAP (5 μg each) as indicated at room temperature for 1 h (right). IB, immunoblot; IP, immunoprecipitation; re., recombinant.