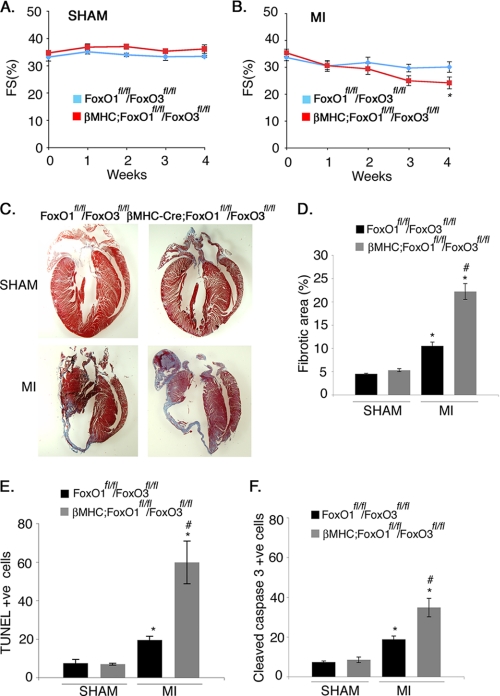
FIGURE 4.
Cardiomyocyte-specific loss of both FoxO1 and FoxO3 leads to increased scar formation and cell death with attenuated cardiac performance following MI. A and B, echocardiographic analysis of fractional shortening in FoxO1fl/fl/FoxO3fl/fl and βMHC-Cre;FoxO1fl/fl/FoxO3fl/fl mice subjected to sham or MI surgical procedure for 4 weeks. Statistical significance was determined by one-way analysis of variance (*, p < 0.05 versus sham, n = 4–7). C, Masson's trichrome-stained histological sections of hearts from FoxO1fl/fl/FoxO3fl/fl and βMHC-Cre;FoxO1fl/fl/FoxO3fl/fl mice subjected to the sham or MI surgical procedure for 4 weeks. The scar area is shown in blue. D, quantitative representation of C showing significant increased in the percentage of fibrotic area in βMHC-Cre;FoxO1fl/fl/FOXO3fl/fl compared with FoxO1fl/fl/FoxO3fl/fl mice. E and F, quantitative representation of cell death as determined by the immunohistochemical staining with TUNEL or cleaved caspase-3-specific antibody in FoxO1fl/fl/FoxO3fl/fl and βMHC-Cre;FoxO1fl/fl/FoxO3fl/fl mice subjected to the sham or MI surgical procedure for 4 weeks. Cell death evaluated by both TUNEL (E) and cleaved caspase-3 (F) is increased in βMHC-Cre;FoxO1fl/fl/FoxO3fl/fl compared with FoxO1fl/fl/FoxO3fl/fl mice. Significance was determined by Student's t test (*, p < 0.05 versus sham and #, p < 0.05 versus MI, n = 6).