FIGURE 1.
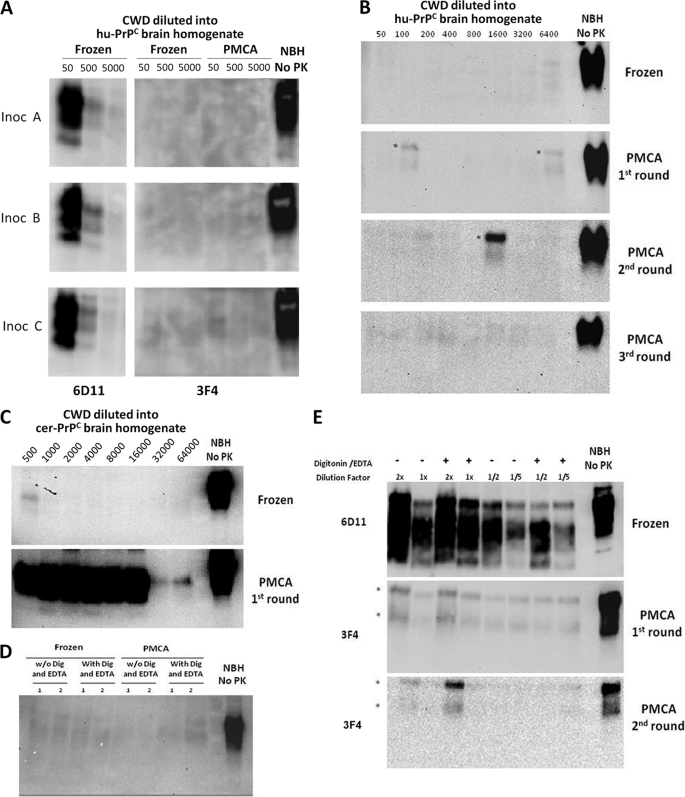
Unsuccessful conversion of human PrPC by CWD PrPSc. A, three different samples of brain homogenates from mule deer naturally affected with CWD were used to attempt conversion of human PrPC (hu-PrPC). 10% CWD brain homogenates were diluted 50-, 500-, or 5000-fold into 10% brain homogenates from transgenic mice expressing the human (129M) PrP gene-coding segment. Samples were either frozen or subjected to 144 cycles of PMCA. Formation of PrPSc was assessed by PK digestion, followed by Western blotting using antibody 3F4, which recognizes human, but not cervid, PrP (22). The amount of cervid PrPSc in the inoculum (Inoc) was checked by Western blotting using antibody 6D11. B, to further attempt conversion of human PrPC with cervid prions, several serial rounds of PMCA were done using standard conditions. As reported previously, three serial rounds of PMCA should lead to ∼100,000-fold amplification of any PrPSc signal present in the sample (24). C, the CWD PrPSc conversion of deer PrPC by PMCA was studied using samples of 10% CWD brain homogenate (inoculum A) that were diluted into 10% brain homogenate prepared from healthy transgenic mice expressing cervid PrPC (cer-PrPC). The formation of cervid PrPSc was assessed by Western blotting using antibody 6D11. D, crossing the cervid-human species barrier was further attempted by adding detergents and EDTA to the conversion buffer, which has been shown to increase PMCA efficiency (37), particularly for human samples (supplemental Fig. 1). E, the CWD inoculum was concentrated by the Sarkosyl procedure described under “Experimental Procedures” to add larger amounts of PrPSc. 2-, 1-, 0.5, or 0.2-fold PrPSc relative to the 10% CWD brain homogenate was added to the human transgenic mouse brain homogenate and subjected to PMCA in either the presence or absence of digitonin (Dig) and EDTA. In the experiments shown in all panels, samples were treated with PK (50 μg/ml), except for the normal brain homogenate (NBH No PK) used as control. The asterisks in B and E represent incomplete digestion of PrPC, which is clearly appreciated because the electrophoretic mobility is identical to that of full-length PrPC.