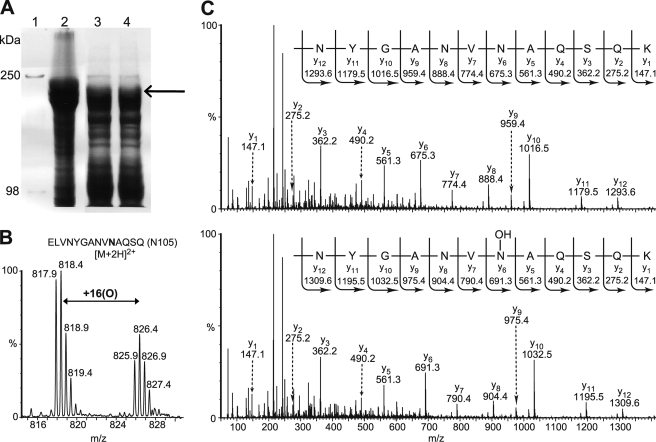
FIGURE 3.
Human erythrocyte ankyrinR is hydroxylated. A, SDS-PAGE analysis shows the purification of endogenous ankyrinR from a human. Isolated erythrocyte ghosts were washed using Triton X-100, the remaining pellet was extracted using 2 m KCl, and the supernatant from the KCl extraction was dialyzed to remove the salt. Lane 1, Mr marker; lane 2, erythrocyte ghost; lanes 3 and 4, dialyzed supernatant from the KCl extraction. The arrow indicates where ankyrinR was identified by tryptic digestion and MS analyses. B, the LC/MS spectrum shows the unhydroxylated ([M+2H]2+ = m/z 817.9) and hydroxylated ([M+2H]2+ = m/z 825.9) forms of the 96–110 tryptic peptide containing Asn-105. C, MS/MS assignment of hydroxylation at Asn-105 in the 96–110 tryptic peptide in human endogenous ankyrinR is shown. A +16-Da mass shift is observed in the y ion series appearing at y6, corresponding to fragments containing Asn-105.