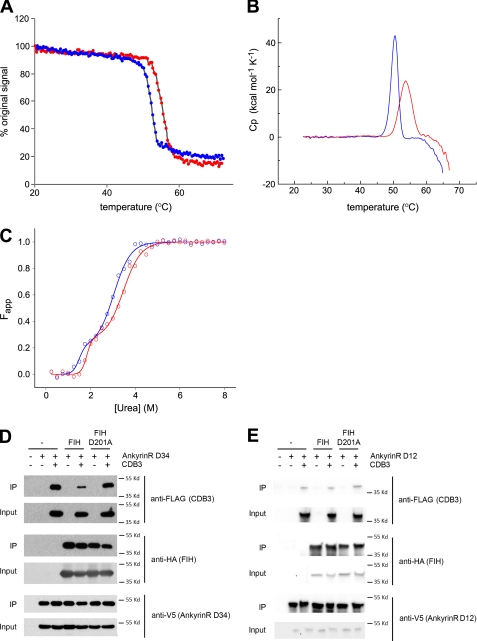
FIGURE 4.
Biophysical effects of D34 hydroxylation. A–C, hydroxylation increases the conformational stability of D34. Data are representative of two independent experiments. A, temperature-induced denaturation of D34 (blue) and D34-OH (red) monitored by CD spectroscopy at 222 nm is represented as the percentage of the original signal at 20 °C. The midpoint of the unfolding transitions (Tm) was 52.2 °C for D34 and 55.3 °C for D34-OH. B, DSC monitored temperature induced denaturation data for D34 and D34-OH after base-line correction and normalization is shown. The melt temperatures obtained were 50.3 °C for D34 and 53.6 °C for D34-OH. C, shown is urea-induced equilibrium unfolding of D34 and D34-OH (open circles) fitted to a three-state model (23) (solid line) using SigmaPlot. The midpoints of unfolding (D50) was obtained from the curve fit as follows: D50 N→I = 1.5 m for D34 and 1.8 m for D34-OH; D50 I→U = 3.0 m for D34, and 3.5 m for D34-OH. D, hydroxylation reduces the interaction of D34 with CDB3. Immunoblot analyses of the interaction of D34 with CDB3 in the presence or absence of FIH overexpression is shown. V5-tagged D34 was immunopurified from HEK293T cells co-expressing FLAG-CDB3 (aa 2–379) in the presence or absence of wild type or D201A HA-tagged FIH. The resultant complexes were immunoblotted for CDB3, D34, and FIH. IP, immunoprecipitate. E, hydroxylation does not alter the interaction between D12 and CDB3. Immunoblot analyses of the interaction of D12 with CDB3 in the presence or absence of FIH overexpression are shown. V5-tagged D12 was immunopurified from HEK293T cells co-expressing FLAG-CDB3 (aa 2–379) in the presence or absence of wild type or D201A HA-tagged FIH. The resultant complexes were immunoblotted for CDB3, D12, and FIH. Cp, specific heat capacity at constant pressure; Fapp, apparent fraction of unfolded protein.