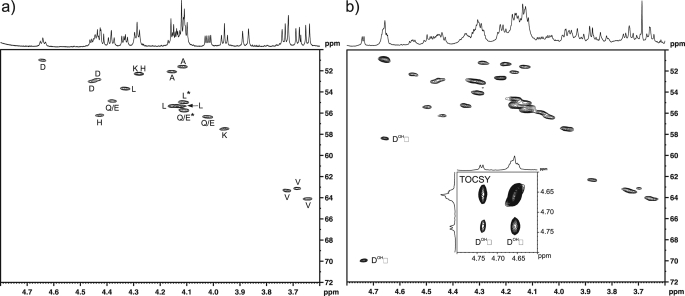
FIGURE 6.
FIH-catalyzed Asp hydroxylation occurs at the β-position. Hydroxylated Peptide 2 (HLEVVKLLLEHGADVDAQDK) was produced by incubation with FIH under standard assay conditions (hydroxylated to ∼75% as assessed by MALDI-TOF analyses), purified by reverse phase HPLC, and analyzed by NMR spectroscopy. a, shown is the α-proton region of the 13C,1H one-bond correlation spectrum (HSQC) of the Asp-substrate Peptide 2 in methanol-d4 with amino acid assignments indicated (Gly residue not included; Gln and Glu resonances have not been differentiated; *, assignments may be interchanged owing to 1H overlap). b, shown is the 13C,1H one-bond correlation spectrum (HSQC) of the HPLC-purified incubation product (both unhydroxylated and the hydroxylated peptide are present). Resonances arising from the α- and β-hydrogens of the hydroxylated Asp residue are indicated, and the 1H,1H correlation arising from the three-bond coupling between these is apparent in the TOCSY spectrum (inset).