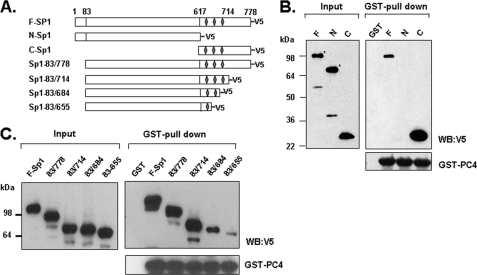
FIGURE 5.
C-terminal domain of Sp1 is required for PC4 interaction. A, schematic representation of the structure of full-length Sp1 (F-Sp1) and its deletion mutants. Three zinc finger domains of Sp1 are shown as diamonds. B and C, GST pull-down assays of PC4/Sp1 interaction with bacterial GST/GST-PC4 and in vitro translated full-Sp1, N-terminal Sp1, C-terminal Sp1, and a series of its deletion mutants including Sp1-(83–778), Sp1-(83–714), Sp1-(83–684), and Sp1-(83–655). The pull-down samples were analyzed by immunoblotting with antibodies against V5 and PC4. (F-Sp1, full-length Sp1 (1–778 aa); N-Sp1, N-terminal Sp1 (1–617 aa); C-Sp1, C-terminal Sp1 (617–778 aa)).