Abstract
Background
The Arabidopsis bypass1 (bps1) mutant root produces a biologically active mobile compound that induces shoot growth arrest. However it is unknown whether the root retains the capacity to synthesize the mobile compound, or if only shoots of young seedlings are sensitive. It is also unknown how this compound induces arrest of shoot growth. This study investigated both of these questions using genetic, inhibitor, reporter gene, and morphological approaches.
Results
Production of the bps1 root-synthesized mobile compound was found to require active root growth. Inhibition of postembryonic root growth, by depleting glutathione either genetically or chemically, allowed seedlings to escape shoot arrest. However, the treatments were not completely effective, as the first leaf pair remained radialized, but elongated. This result indicated that the embryonic root transiently synthesized a small amount of the mobile substance. In addition, providing glutathione later in vegetative development caused shoot growth arrest to be reinstated, revealing that these late-arising roots were still capable of producing the mobile substance, and that the older vegetative leaves were still responsive.
To gain insight into how leaf development responds to the mobile signal, leaf development was followed morphologically and using the CYCB1,1::GUS marker for G2/M phase cells. We found that arrest of leaf growth is a fully penetrant phenotype, and a dramatic decrease in G2/M phase cells was coincident with arrest. Analyses of stress phenotypes found that late in development, bps1 cotyledons produced necrotic lesions, however neither hydrogen peroxide nor superoxide were abundant as leaves underwent arrest.
Conclusions
bps1 roots appear to require active growth in order to produce the mobile bps1 signal, but the potential for this compound's synthesis is present both early and late during vegetative development. This prolonged capacity to synthesize and respond to the mobile compound is consistent with a possible role for the mobile compound in linking shoot growth to subterranean conditions. The specific growth-related responses in the shoot indicated that the mobile substance prevents full activation of cell division in leaves, although whether cell division is a direct response remains to be determined.
Background
Plants synthesize a wide array of metabolites, and a major goal of metabolomics is to identify natural plant metabolites and their associated functions (reviewed in [1-3]). Recent advances facilitating identification of metabolites [4,5] have led to identification of groups of metabolites that correlate with important plant traits, such as growth rate and biomass [6,7], and identified metabolic regulators such as leucine [8]. However, how specific metabolites other than characterized hormones function in signaling and development is largely unknown. One approach to learning about alternate signaling molecules is to study mutants with signaling-related defects.
The Arabidopsis bypass1 (bps1) mutant might be an important tool for identifying a metabolite functioning as a long-distance signal. The bps1 mutant produces small abnormal roots and shoot development arrests soon after germination. This phenotype is linked to a mobile substance as the bps1 mutant root is necessary to induce arrest of bps1 shoots, and in graft chimeras, the bps1 root is sufficient to induce arrest of the wild-type shoot [9]. These observations led to a model featuring BPS1 as a negative regulator that was required to prevent the excess production of a mobile substance. The mobile compound appears to be novel, and its synthesis requires carotenoid biosynthesis [10]. The pathway producing the bps1 mobile compound appears to be conserved in plant lineages, as knock-downs of conserved BPS-like genes in tobacco produced similar phenotypes [11]. Critical questions include whether this mobile compound is an endogenous developmental regulator, and how it modifies shoot growth.
Control over shoot branching by a root-derived signal has been elegantly analyzed in pea, rice, and Arabidopsis [12-15]. In these systems, mutations disrupting biosynthetic enzymes lead to reduced production of a mobile compound that controls auxin transport in the shoot [16,17]. Recently, this substance was identified as strigolactone [18,19]. Additional unknown root-to-shoot signals have been implicated by studies of drought (reviewed in [20]), soil compaction [21], nutrient depletion [22-24] and low-fluence UV-B light [25]. The identities of the mobile compounds elicited by these treatments are unknown; it is also unknown whether the bps1 mobile substance is related to any of these pathways, but its root-to-shoot mobility make it an attractive candidate.
It is also possible that the bps1 mobile compound could instead be an intermediate molecule that normally doesn't accumulate. For example, a biosynthetic pathway might be blocked in bps1 mutants, resulting in build-up of a precursor that happens to be mobile, and happens to have biological activity. For example, in superroot1 mutants, a defect in glucosinolate biosynthesis causes a build-up of precursors that spills over into auxin biosynthesis, resulting in a high-auxin phenotype [26].
Here, we evaluate the conditions under which bps1 roots produce the mobile compound, and the characteristics of shoots undergoing arrest from this substance. We find that bps1 roots produce and transport the mobile substance in actively growing roots, but that arrest of cell division leads to cessation of signaling to the shoot. Shoot responses include growth cessation, and in particular, arrest of cell division.
Results
The bps1 root: shoot growth inhibition requires root growth
The central feature of bps1 mutants is that a growth-arresting mobile compound arises in the root [9]. However, the experimental basis for this assignment required wounding, and it was only tested in very young seedlings. To expand our understanding of the root's role in producing the bps1 signal, we examined how leaf development responded when bps1 root growth and development was blocked after embryogenesis. Post-embryonic root growth and development requires glutathione (GSH, [27]). Root development can therefore be blocked by either supplying germinating seeds with L-buthionine sulfoximine (BSO), an inhibitor of γ-glutamylcysteine synthetase, or by generating double mutants between bps1 and root meristemless1-1 (rml1-1), which has a defect in the gene encoding γ-glutamylcysteine synthetase and lacks post-embryonic root development [27,28].
In agreement with previous publications, the roots of BSO-treated wild type appeared to arrest development at germination. In addition, these plants produced small, but flattened, leaves with distinct blade and petiole, a modest reduction in leaf vein pattern, and bleached cotyledons (Figure 1). By contrast, bps1 mutants grown on BSO-supplemented medium produced leaves with two distinct shapes (Figure 1). The first leaf pair was small, radially symmetric, and contained only a single unbranched vein, while subsequently produced leaves were broad, flat, showed distinct blade and petiole, and contained both primary and secondary veins. This partial rescue of leaf development in BSO-treated bps1 mutants suggested that post-germination arrest of the bps1 root led to reduced synthesis of the root mobile signal.
Figure 1.
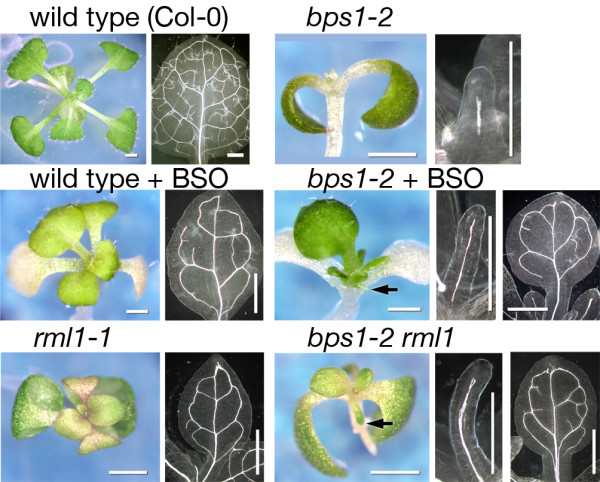
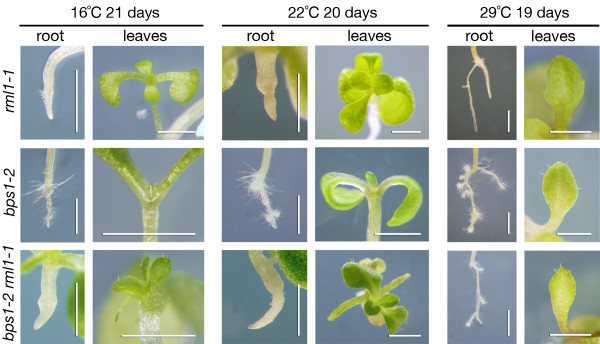
Sustained synthesis of the bps1 mobile compound requires post-embryonic root growth. The top row shows wild type seedlings with their broad flat leaves and highly interconnected leaf veins, and bps1-2 mutants, with their very small radial leaf primordia and incomplete primary vein. The second row shows the effect of BSO-induced root arrest on wild type and bps1 mutants. In the wild type, shoots of BSO-grown plants had chlorotic cotyledons and small leaves with modestly reduced vein patterns. The shoots of bps1-2 mutants growth on BSO-containing media produced two types of leaves: the first leaf pair (black arrow) were elongated but radial, and contained only a single vein, while leaves that arose later were broad, flat, and produced more complex vein patterns. The third row shows rml1-1 and bps1-2 rml1-1 double mutants. The rml1-1 mutants were slightly smaller than the wild type and they produced slightly narrow, pointed leaves with modestly reduced patterns of veins. The bps1-2 rml1-1 double mutants produced two types of leaves: the first leaf pair (black arrow) were radialized but elongated, and contained a single vein, while later leaves were broad, flat, and produced complex vein patterns. Size bars: 500 μm.
Similarly, the roots of rml1-1 mutants appeared to arrest development at germination, and they produced small, flattened leaves with distinct blade and petiole (Figure 1). Growth of rml1-1 mutants on GSH-supplemented medium restored post-embryonic root growth, as reported previously [27] (Table 1). The bps1 single mutants and F2 seedlings derived from rml1-1/+ bps1-2/+ parents, grown on GSH-supplemented media, were indistinguishable from bps1 controls (Table 1). By contrast, F2 seedlings derived from rml1-1/+ bps1-2/+ parents grown on standard growth medium (lacking GSH), segregated for four different phenotypes: wild type; rml1-1; bps1-2; and a phenotype similar to BSO-grown bps1 mutants (Figure 1). This last phenotype appeared at numbers consistent with it being the rml1-1 bps1-2 double mutant (Table 1). As with rml1-1, the bps1 rml1-1 double mutants produced roots that showed no sign of post-embryonic cell divisions. However, their first pair of leaves were radial and contained a single unbranched vein; these were similar to the bps1 single mutant, but much longer (Figure 1). Strikingly, leaf 3 and subsequently produced leaves were broad and flattened, with distinct petiole and blade, and contained both primary and secondary veins, much like the leaves of rml1-1 single mutants and BSO-treated bps1 (Figure 1). Thus, these data indicate that post-embryonic root growth and development is required for continuous production and delivery of the leaf-arresting substance and further support the root as the source of this molecule [9].
Table 1.
The bps1 shoot phenotype requires post-embryonic root development
| Shoot Phenotypes Observed | |||||||
|---|---|---|---|---|---|---|---|
| Plants analyzed | GSH | Total (n) |
wild type (n) |
rml1 (n) |
bps1 (n) |
bps1 rml1 (n) |
χ2 |
| rml1-1 | - | 240 | 189 | 51 | 0 | 0 | 1.8a |
| rml1-1 | + | 70 | 70 | 0 | 0 | 0 | n/a |
| bps1-2 | - | 224 | 164 | 0 | 60 | 0 | 0.381a |
| bps1-2 | + | 111 | 85 | 0 | 26 | 0 | 0.147a |
| rml1-1 bps1-2 F2 | - | 1258 | 728 | 236 | 210 | 84 | 3.793b |
| rml1-1 bps1-2 F2 | + | 590 | 437 | 0 | 153 | 0 | 0.2734a |
n/a, not applicable
a Critcal χ2 values at 95% confidence is 3.841 when df = 1.
b Critical χ2 values at 95% confidence is 7.815 when df = 3.
Because imposing a GSH deficit (using either BSO or rml1-1) led to partial rescue of the bps1 shoot phenotype, it was a formal possibility that the bps1 root-derived compound could be GSH itself. To test this, we supplied GSH to excised bps1 shoots, and monitored subsequent leaf development. Typically, root excision leads to partially rescued shoot development in approximately 75% of bps1 single mutants [9]. We reasoned that if the mobile signal was GSH, supplying it to bps1 mutants following root excision would reduce the number of bps1 mutants that were rescued by root excision. However, supplying GSH did not diminish shoot developmental rescue, and no regimen of GSH provision (prior to cut, after cut, or both prior and after cut) yielded a statistically significant reduction of developmental rescue. Moreover, shoot rescue resulted in leaves reaching similar sizes, whether or not excised shoots were supplemented with GSH (Table 2). These data indicated that the mobile compound was not GSH.
Table 2.
Analysis of GSH as a candidate for the BPS1-regulated signal
| Growth Medium | Total bps1 with excised root (n) | Percent producing broad leaves with distinct blade and petiole (n) | |
|---|---|---|---|
| Pre-excision | Post-excision | ||
| GSH- | GSH- | 31 | 77% (24) |
| GSH- | GSH+ | 40 | 83% (33) |
| GSH+ | GSH- | 27 | 70% (19) |
| GSH+ | GSH+ | 34 | 94% (32) ** |
** The number of plants that produce leaves under GSH+/GSH+ conditions is statistically greater than the number that produces leaves under GSH-/GSH-conditions (p value = 0.009).
Arrested roots provide a transient source of the bps1 signal
Arrest of post-embryonic root growth in bps1 caused strikingly different responses in the first leaf pair as compared to leaf three. Both BSO-grown bps1 mutants and rml1 bps1 double mutants initially produced a pair of radialized leaves, yet rescued development was observed in subsequently produced leaves (Figure 1). In addition, the rml1-1 bps1-2 first leaf pair was consistently larger than that of BSO-grown bps1-2 mutants. These results contrast to root excision (carried out at day 4), where the strongest rescue was observed in the first leaf pair [9], and suggest that the arrested bps1 root might be a transient source of the bps1 mobile compound.
We tested this possibility using the bps1 temperature dependent phenotype [9]. We compared the first leaf pair of bps1 rml1-1 double mutants grown at 16, 22, and 29°C. For bps1 single mutants, leaf development is temperature dependent: severe arrest occurs at low temperatures, and small but flattened leaves are produced at high temperatures. Thus, if the radialized first leaf pair of bps1 rml1-1 double mutants was due to the bps1 mobile signal, then we expected its development to be similarly dependent on growth temperature.
Growth of bps1 rml1 double mutants at 16°C led to production of a narrow radially-shaped first leaf pair that was much longer than the bps1-2 control (Figure 2). In bps1 rml1 double mutants grown at 22°C, the first leaf pair was long, but very narrow. Its narrowness was similar to the age-matched bps1 single mutant, but it was much longer. Finally, in bps1 rml1 double mutants grown at 29°C, the first leaf pair was flattened and had a distinct blade, very similar to the bps1 control. The similar effects of growth temperature on the first leaf pair of bps1 single and bps1 rml1 double mutants indicates that the shape of the first leaf pair is due to the bps1 mobile root-derived compound. This suggests that the first leaf pair of bps1 rml1 double mutants was exposed to the root-derived compound, while the later-arising rescued leaves were not.
Figure 2.
Temperature responsive phenotype of the bps1 rml1 first leaf pair supports exposure to the bps1 mobile compound during development. In bps1 single mutants, leaf development arrests early at low temperature (16°C), while growth at higher temperatures results in progressively more leaf growth. In bps1-2 rml1-1 double mutants, development of the first leaf pair responds similarly to growth temperature. When grown at 16°C, the first leaf pair was small and radial, growth at 22°C led to much longer, but still very narrow leaves, and growth at 29°C led to small but broad leaves. Note that the rml1 single mutant also showed enhanced root growth at the elevated temperature.
Competence to synthesize and respond to the bps1 signal is retained in older seedlings
Although the molecular target of the bps1 root-derived mobile compound is unknown, root-dependent arrest of early shoot development in bps1 seedlings indicates that the target is present at this early stage. However, we do not know if the molecular target is present later in development, nor do we know whether an older root retains the capacity to synthesize the mobile compound. To test this, we followed up on the observation that rml1 root development is rescued by supplying glutathione (GSH) [27]. We reasoned that supplying GSH to an older bps1 rml1 double mutant might restore growth of a bps1-like root. If this root retained the ability to synthesize and deliver the mobile compound, and its molecular target was present in older shoots, then we would expect to observe arrested leaf growth.
Seeds segregating for both bps1 and rml1-1 were plated on standard growth media, and at 10 days, 90 seedlings with rml1 root phenotype were transferred to GSH-supplemented medium (approximately 22-23 were expected to be bps1 rml1 double mutants). At 18 days after transfer (28 total days), we analyzed their phenotypes. Most of the plants looked the same as rml1-1 controls; they produced normal-appearing roots and large flat leaves (Figure 3). However, 18 of the seedlings produced roots that were short, blunt, and very swollen, and had produced lateral roots somewhat similar to bps1. These plants also produced shoots with variable leaf shapes. Their first leaf pair was long and radial, the subsequently produced 5-9 leaves appeared flat (partially rescued), and with distinct petiole and blades. Finally, the newest arising leaves were short and radially shaped (bps1-like). This range of leaf phenotypes is consistent with restored synthesis and delivery of the bps1 mobile compound upon induction of root growth, and response in these later-arising vegetative leaves. These results indicate that roots retain the capacity to synthesize and deliver the bps1 mobile substance to the shoot, and that the shoots of older seedlings retain the ability to respond.
Figure 3.
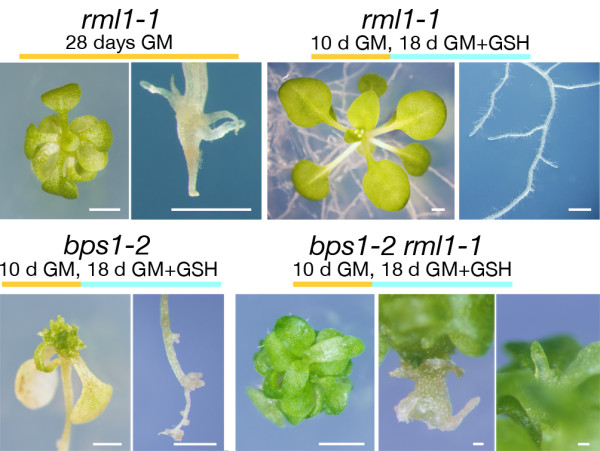
Restored root growth in bps1-2 rml1-1 plants reinstates arrest of leaf development. Top row: rml1-1 grown for 28 days on normal growth medium (GM) (left) and or transferred to GSH-supplemented GM at day 10 (right). The small stunted root is rescued by transfer to GSH (+). Bottom row: bps1-2 (left) and bps1-2 rml1-1 (right), both transferred from GM to GM+GHS at 10 days. The bps1 single mutants produced small roots and narrow radialized leaves. The bps1-2 rml1-1 double mutants showed a novel phenotype. The roots enlarged radially, they produced a few enlarged lateral-root-like organs, and arrested leaf primordia accumulated at the shoot apex. Size bars = 1 mm except for bps1-2 rml1-1 roots and leaf primordia, where bars = 0.1 mm.
The bps1 mobile compound: synthesis and delivery require neither the phloem nor endodermis
We next developed double mutants that combined bps1 with altered phloem development (apl), shortroot (shr), and scarecrow (scr) mutants. The apl mutant lacks phloem, shr lacks endodermis, and scr replaces endodermal and cortical cell layers with a single layer of mixed identity [29-33]. We predicted that if the phloem or endodermis were the sole site of synthesis of the bps1 mobile compound, or required for its transmission, then leaf development would be at least partially restored in the double mutants.
The bps1 apl double mutants showed an arrested shoot phenotype that was indistinguishable from bps1 (Figure 4), indicating that phloem was dispensable for synthesis and delivery of the bps1 mobile compound, at least in these very small mutants. Similarly, both shr bps1 and scr bps1 double mutants resembled the bps1 single mutant (Figure 4, Table 3). Taken together, these data indicate that normal root development, including formation of the phloem and the endodermis, is not required for production and delivery of the bps1 signal.
Figure 4.
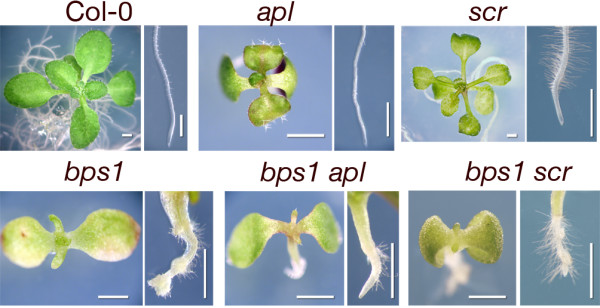
Neither phloem nor endodermal cell types are required for production or transmission of the bps1 mobile compound. Top row: shoot and root phenotypes of 15-day Col-0 (left), the phloem-deficient apl (middle), and scarecrow (scr, right). Bottom row: shoot and root phenotypes of bps1 (left), the bps1 apl double mutant (center), and bps1 scr double mutant (right). The double mutants show a leaf arrest phenotype that is similar to that of bps1, indicating that the root-derived mobile compound is still synthesized and transmitted to the shoot. Seedlings shown here were grown at 22°C and photographed at 15 days. Size bars: 1 mm.
Table 3.
shr and scr radial patterning defects do not suppress the bps1 shoot phenotype
| Shoot Phenotypes Observed | |||||||
|---|---|---|---|---|---|---|---|
| Plants analyzed | Total (n) |
wild type (n) |
scr (n) |
shr (n) |
bps1 (n) |
Double mutant (n) |
χ2 |
| scr3-9 bps1-2 F3 | 721 | n/a | 545 | n/a | n/a | 176 | 0.134a |
| shr bps1-2 F2 | 1040 | 576 | n/a | 194 | 270 | n/a | 0.528b |
| shr | 167 | 129 | n/a | 38 | n/a | n/a | 0.449a |
| bps1-2 | 191 | 146 | n/a | n/a | 45 | n/a | 0.211a |
n/a, not applicable
a Critcal χ2 values at 95% confidence is 3.841 when df = 1.
b Critical χ2 values at 95% confidence is 5.991 when df = 2.
Shoot responses to the bps1 mobile root-derived compound
The reversible arrest of shoot development in bps1 mutants correlates with a loss of auxin responses [9], but the underlying mechanism of arrest is unknown. To broaden our understanding of shoot responses in bps1, we carried out a series of time-course analyses where we analyzed leaf size, shape, the distribution of dividing cells, and stress responses (necrotic lesion formation and appearance of ROS).
Dividing cells were identified using the CYCB1;1::GUS reporter, a cell cycle reporter expressed in cells at the G2/M phase [34]. Patterns of CYCB1;1::GUS expression in Arabidopsis are well characterized; early leaf development shows a nearly uniform distribution of GUS-staining (i.e. dividing) cells, while later in development cell divisions become restricted to the leaf base and provascular tissue [35] (Figure 5). In bps1 mutants, the three-day leaf primordia largely matched wild type in terms of size, shape and CYCB1;1::GUS expression patterns, however there were pronounced differences by day four. The four-day wild type leaf was much larger than that of bps1 and CYCB1;1::GUS-staining cells were distributed throughout, while the small four-day bps1-2 leaf had only a few CYCB1;1::GUS-staining cells. At day five, the wild-type leaf started to show distinct lamina expansion, and a slight tendency for there to be more GUS-staining cells toward its proximal end. By contrast, the five-day bps1 leaf showed no sign of lamina expansion, and few CYCB1;1::GUS-staining cells. The six-day wild-type leaf showed a strong reduction of CYCB1;1::GUS-staining cells at the distal end, and was much larger than the five-day wild-type leaf, while the corresponding bps1 leaf was largely unchanged. By day seven, the wild-type leaf was even larger and the few CYCB1;1::GUS expressing cells were at the leaf base. Similarly, the bps1 seven-day leaf had only a few CYCB1;1::GUS-staining cells, and most were restricted to the leaf base. This analysis revealed a fully penetrant leaf arrest phenotype. In addition, despite the striking reduction in numbers of dividing cells, the apical/basal spatial control of cell divisions appeared to be intact.
Figure 5.
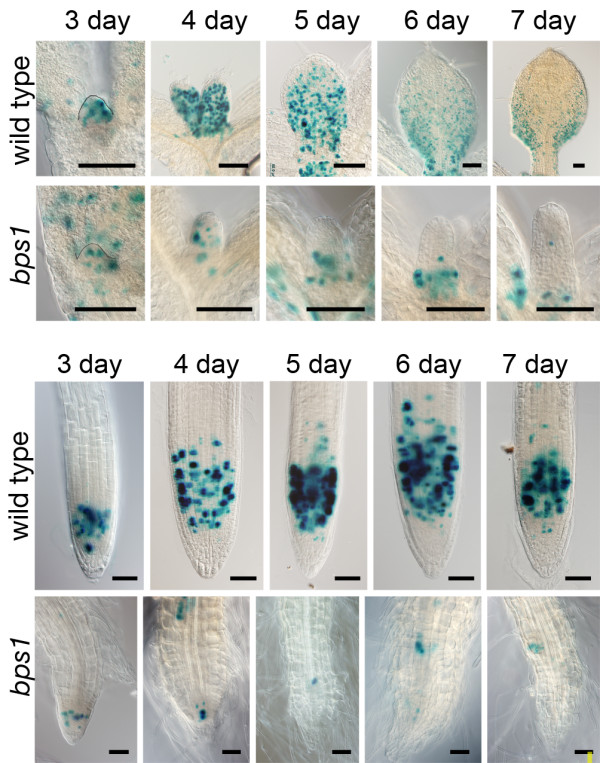
Growth and cell cycle progress are diminished in bps1 leaves and roots. GUS stained tissue from bps1 and wild-type plants carrying CYCB1;1::GUS transgene. Top set: representative leaves, harvested at the same time daily (days three through seven). Bottom set: roots from the same time points. For both organs, severe effects on growth and development were obvious in bps1 by day four, and included dramatic reduction in the number of G2/M phase cells. Size bar = 0.1 mm (leaves), and 0.05 mm (roots).
While carrying out this analysis of leaf development, we also compared patterns of CYCB1;1::GUS-staining in roots (Figure 5). In the wild type, CYCB1;1::GUS-staining patterns were restricted to the root meristem, as has been described previously [36], and a similar pattern was observed between days three and seven. By contrast, at all time points, the bps1 root had fewer CYCB1;1::GUS-staining cells.
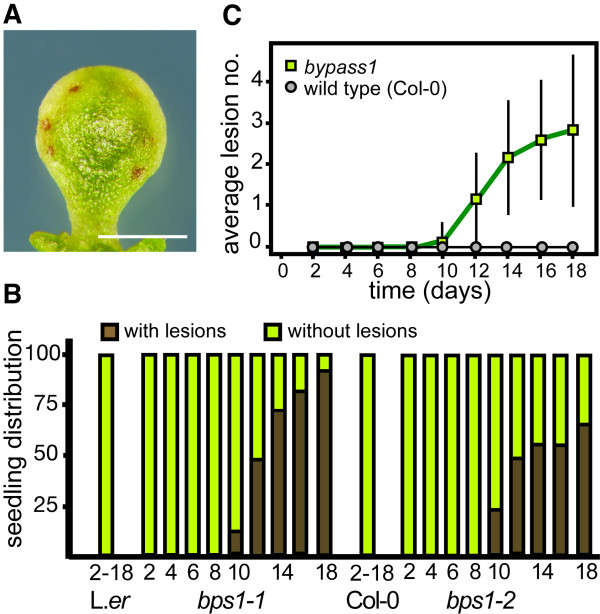
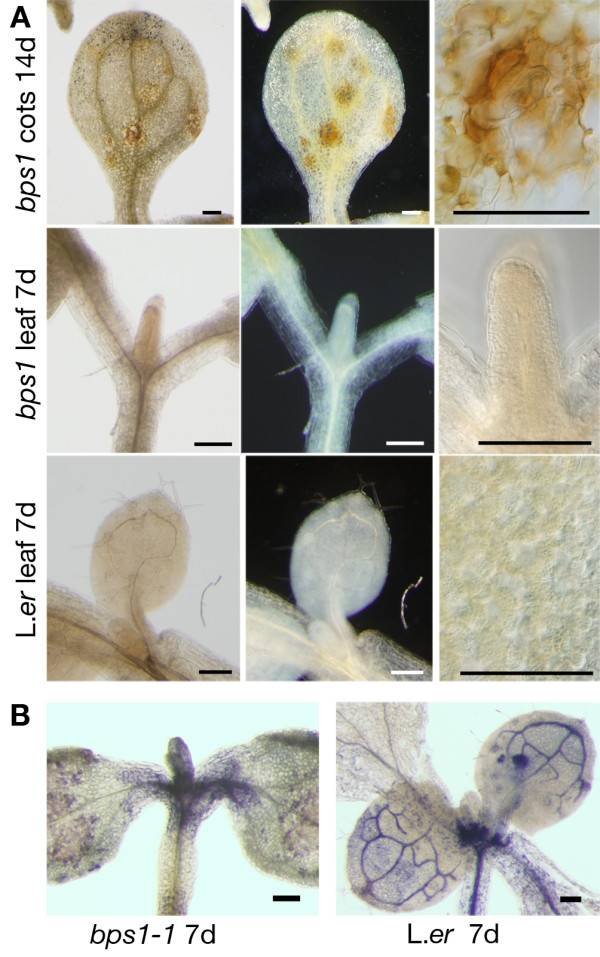
The bps1 mutant analysis revealed occasional necrotic lesions on bps1 cotyledons (Figure 6A). To assess a possible relationship between these lesions and leaf arrest, we carried out another time-course analysis, this time examining wild type and bps1 mutants for necrotic lesion formation. In bps1 mutants, necrotic lesions began to appear between 8 and 10 days. They were restricted to cotyledons, and never observed on leaves, hypocotyls, or roots, and necrotic lesions were never observed on the wild type (Col-0 or L.er) (Figure 6B). More bps1-1 seedlings formed necrotic lesions than bps1-2, and by day 18, 92% of the bps1-1 plants had at least one necrotic lesion. The average lesion number per plant was highly variable, and increased over time (Figure 6C); by day 18 the bps1-2 mutants had between zero and seven necrotic lesions. Both bps1-1 and bps1-2 are null alleles [1], and so we attribute the difference in lesion formation to their genetic backgrounds (Col-0 for bps1-2 and L.er for bps1-1).
Figure 6.
Necrotic Lesion formation is a late and not fully penetrant phenotype in bps1 mutants. (A). 14-day bps1-1 cotyledon with necrotic lesions. (B). Onset and penetrance of necrotic lesion formation. Green bars represent the percent of plants with no lesions, and brown bars represent the percent with one or more necrotic lesion. Neither the L.er nor Col-0 wild type produced any necrotic lesions, whereas for both bps1-1 and bps1-2 mutants formed necrotic lesion starting between day 8 and 10, after which lesion number increased steadily. N = 84 (L.er), 140 (bps1-1), 48 (Col-0), 80 (bps1-2) (C) Average number of lesions per seedling. The number of lesions per seedling is depicted as a function of time, and bars show standard deviation. size bar = 1 mm.
Because lesion formation is typically preceded by reactive oxygen species (ROS) [37-40], we compared ROS in bps1 and wild type shoots using diaminobenzadine (DAB) to assay for hydrogen peroxide (H2O2) and nitroblue tetrazolium (NBT) to assay for superoxide. Because the bps1-1 allele showed a more robust necrotic lesion phenotype, these analyses used bps1-1 and L. er. Both staining procedures produced a strong reaction in the vascular tissue, consistent with a role for ROS in lignification [41,42]. We found H2O2 in the 14-day bps1 cotyledons, typically in positions surrounding the developing necrotic lesions (Figure 7A), but did not observe any nonvascular staining in the wild type cotyledon (data not shown). Additionally, we did not detect H2O2 in bps1 leaves. Similarly, superoxide was primarily associated with vascular tissue in wild type leaves, and it was nearly absent from the leaves of bps1 mutants (Figure 7B). Because accumulation patterns of these two ROS were similar for the wild type and bps1 mutants, severe oxidative stress does not appear to cause the bps1 leaf developmental arrest.
Figure 7.
ROS is not increased in arrested bps1 leaves. (A). Hydrogen peroxide, visualized using DAB, was found in necrotic lesions and associated with vascular tissue, but it was not elevated in the bps1 leaf. (B) Superoxide, visualized using NBT staining, was found associated with vascular tissue, but it was not elevated in the bps1 leaf. Bars = 200 μm.
Discussion
Physiological studies have implicated long distance signaling as a link between the development and physiology of roots and shoots [20]. However, only a small number of long-distance signaling pathways have been verified molecularly. In Arabidopsis bps1 mutants, the non-cell-autonomous activity of mutant roots suggests that BPS1 might function to limit the synthesis of a root-derived mobile signaling molecule [9].
Capacity to Synthesize the BPS1 Mobile Compound
A central feature of bps1 mutants is that the root is the source of a biologically active mobile compound, which we refer to as the bps1 signal. Here, we extended our understanding of the conditions under which the mutant root produces this compound. Previously we showed that cutting off the root led to rescue of the first leaf pair [9]. Indeed, we have now found that arresting post-embryonic bps1 root growth also resulted in rescue of leaf development. However, in contrast to root excision, the first leaf pair was only mildly rescued, and strong rescue was delayed until leaf three. These observations indicate that the bps1 root, despite post-embryonic arrest, retained a transient ability to supply the bps1 signal to the shoot.
We used two related approaches to arrest post-embryonic root growth: we caused arrest through the depletion of GSH either genetically (using the rml1-1 mutant) or chemically (using BSO). In both cases, the first leaf pair in GSH-depleted bps1 was larger than that of untreated bps1 mutants, and the first leaf pair of bps1 rml1-1 double mutants were consistently larger than that of BSO-grown bps1. Here, a larger leaf size probably reflects an earlier block to GSH synthesis in the mutant, and therefore an earlier reduction in bps1 signal synthesis.
Similarly, we found that restoring development of bps1 roots (by GSH provision to bps1 rml1-1 seedlings) reinstated arrest of leaf development. The extended capacity to produce and respond to the mobile compound is in line with physiological studies of drought-evoked long distance signaling, which has been documented in diverse plants, and at varying developmental stages [4].
A possibly less obvious question is why growth-arrested roots (i.e. bps1 rml1-1 double mutants and BSO-grown seedlings) show a decreased ability to arrest shoot growth. One possibility is that bps1 signal synthesis has a direct requirement for GSH. Alternatively, either synthesis or transmission to the shoot requires active root growth and cell division.
The maintenance of shoot arrest in apl bps1 double mutants is consistent with a link to root growth. Although apl mutants have determinate roots [29], growth ceases later than for rml1-1 or BSO-treated plants, and the apl bps1 analysis was carried out prior to evidence of root cell division arrest. However, if root growth is a requirement for bps1 signal synthesis, then we would need to be able to explain constitutive synthesis of bps1 signal in bps1 mutants, which show primary root arrest soon after germination. One possibility is that synthesis is sustained by lateral roots, which initiate repeatedly. Alternatively, bps1 roots (including the primary) expand radially, and this radial growth might also sustain synthesis of the bps1 signal.
bps1 signal transmission
Movement of the bps1 signal from the root to the shoot is likely to use the plant's vascular system. Two vascular tissues are specialized for long-distance movement: the phloem, which transports photosynthate, and also mRNAs and proteins; and the xylem, which primarily transports water and dissolved nutrients. Here, we found that the shoot undergoes arrest in bps1 apl double mutants, which lack phloem [29]. The simplest conclusion is that the bps1 signal moves in the xylem. However, this conclusion is not definitive, because the very small size of bps1 apl double mutants doesn't preclude movement by diffusion.
Shoot responses to the mobile bps1 signal
The small leaf size and reduced number of CYCB1;1::GUS expressing cells are a fully penetrant bps1 phenotype. Strikingly, although reduced in number, the pattern of CYCB1;1::GUS-expressing cells mimicked the wild type pattern: leaf primordia showed an even distribution of diving cells, but as the mutant leaves matured, dividing cells were restricted to the base of the leaf. The retention of a normal pattern of dividing cells shows that some aspects of leaf developmental programming persist in bps1 mutants. This result hints that instead of altering development, the bps1 signal might instead disrupt the link between development and cell cycle control.
Another phenotype in bps1 mutants is the formation of necrotic lesions. These were late-appearing and not fully penetrant. Necrotic lesions have been observed in a wide range of Arabidopsis mutants. These include plants with defects in syntaxin genes [43], and mutants with defects in the cytochrome P450 gene CYP83B1, which results in excess auxin synthesis [44]. Necrosis is typically associated with plant defense responses, and can be a secondary consequence of elevated expression of defense genes, such as observed in the developmental mutant asymmetric leaf 1 [45] and in response to phosphate deficiency [46,47].
Conclusions
The results presented here support the phenomenon of shoot arrest by a root-derived molecule in bps1 mutants. A key question raised by discovery and characterization of this mutant is whether the bps1 mutation exposes a novel root-to-shoot signaling molecule or a metabolic intermediate with toxic effects on shoot development. The crucial difference between these two concepts is that a novel root-to-shoot signaling molecule would be present in the wild type, while a metabolic intermediate would only accumulate in bps1 mutants. Because the synthesis of the root-derived molecule requires post-embryonic root development and aerial organs appear to arrest growth prior to showing any signs of toxicity (necrosis), we tend to favor the hypothesis that bps1 reveals a novel root-to-shoot signaling pathway. A full resolution of this issue awaits biochemical identification of this mobile molecule. Regardless of the nature of the root-derived compound, it should be pointed out that under either scenario the bps1 mutation has unveiled a molecule with potent biological activity. Despite the impact of root-to-shoot communication on plant productivity, the molecular mechanisms involved are poorly understood. The bps1 mutation could be utilized as a tool to begin to dig into the pathways that both synthesize and respond to root-derived growth modulators.
Methods
Plant Growth
All seeds were cold-shocked for 2-4 days in darkness at 4°C, and most grown in 24 hour light at the 22°C, unless noted otherwise. Growth media composition is 0.5X MS salts (Caisson labs), 1% sucrose, 0.5g/l MES, pH 5.8, 0.8% phytoblend agar (Caisson Laboratories). Seedlings were grown in Conviron TC30 growth chambers under light and temperature regimes as described.
Plant Materials
Mutant alleles used: bps1-2 (Col), bps1-1 (L.er), rml1-1 (Col, received from Z.R. Sung), scr-3 (Col, CS3997), shr (Col, SALK_002744), apl (Col, received from M. Bonke), and CYCB1;1::GUS seeds were received from J.L. Celenza.
GUS Staining
The CYCB1;1::GUS transgene was crossed into both bps1-1 and bps1-2, and F3 lines homozygous for the transgene and segregating for bps1 were identified. We plated these lines (and control wild-type transgenic) on normal growth media, and subjected them to a 2-7 day cold shock (4°C). Each day, plates were transferred to a 22°C growth chamber. GUS staining followed previously published protocols [48].
Conditional Root Arrest
Arrest of roots using BSO was carried out by making our standard GM (above), and supplementing it to 2.5 mM BSO (DL-Buthionine-[S,R]-sulfoximine, Sigma), and bps1-2 rml1-1 double mutants were generated by standard methods. For both BSO and rml1 experiments, plants were grown in short day (8 hours light/16 hours dark) at 22°C. To reinstate root growth of rml1-1, we supplemented the media to 750 μM glutathione (Acros Organics) [27]. To test whether the number of plants producing broad leaves upon root excision in the presence of GSH was statistically different from that observed in the absence of GSH (Table 2), we performed hypothesis testing for proportions using the Z-score method. If GSH were the root-shoot signal, we would predict that the number of plants forming broad leaves under GSH+ conditions would be less than under GSH-conditions (the null hypothesis). The statistical tests indicate that the number of plants producing leaves in the presence of GSH is not less than or equal to the number producing leaves in the absence of GSH. Because the calculated P value is low, we must reject the null hypothesis in support of the alternative hypothesis that the number of plants producing leaves under GSH+ conditions is greater than GSH-conditions. This indicated that the root-to-shoot signal is not GSH.
Stress symptom analyses
Necrotic lesion formation was assessed by a visual inspection of bps1 and wild type seedlings. All organs of the investigated seedlings were examined on alternate days. To visualize patterns of H2O2 in seedlings (wild type and bps1), we used 3,3'-diaminobenzidine (DAB) staining as described [49,50]. We infiltrated 0.1% (W/V) DAB (Sigma), pH3.8, and allowed staining to progress for 4-6 hours. After staining, samples were cleared in 70% ethanol, and then transferred to 40% w/v glycerol, mounted on glass slides, and examined on Olympus BX50 and Olympus SZX16 microscopes. Visualization of superoxide patterns used nitroblue tetrazolium staining protocols as described [51,52].
Authors' contributions
JMVN carried out rml1 and BSO root arrest experiments and the apl bps1, shr bps1, and scr bps1 double mutant analyses. CM carried out analyses of ROS, characterized the lesion formation phenotype, and analyzed shoot phenotypes following resumption of root development. LES carried out the pCYCB1;1::GUS analyses. LES and JMVN planned the project together, and the manuscript was primarily written by LES with assistance from JMVN. All authors read and approved the final manuscript.
Contributor Information
Jaimie M Van Norman, Email: jaimie.vannorman@duke.edu.
Caroline Murphy, Email: c.murphy@utah.edu.
Leslie E Sieburth, Email: sieburth@biology.utah.edu.
Acknowledgements
We would like to thank Dong-Keun Lee and Emma Adhikari for useful discussions of the work and for proofreading. This project supported by the National Research Initiative competitive grant no. 2008-35304-04488 (to LES) from the USDA National Institute of Food and Agriculture, by award IOB-0922288 (to LES) from the National Science Foundation, and an award from NSF-supported BioURP award to CM and NIH training grant support to JMVN (NIH training grant number 5 T32 GM007464).
References
- Last RL, Jones AD, Shachar-Hill Y. Towards the plant metabolome and beyond. Nat Rev Mol Cell Biol. 2007;8:167–174. doi: 10.1038/nrm2098. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Akiyama K, Sakata A, Kuwahara A, Otsuki H, Sakurai T, Saito K, Hirai MY. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant Cell Physiol. 2009;50:37–47. doi: 10.1093/pcp/pcn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano K, Kurihara Y, Seki M, Shinozaki K. 'Omics' analyses of regulatory networks in plant abiotic stress responses. Curr Opin Plant Biol. 2010;13:132–138. doi: 10.1016/j.pbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Giavalisco P, Hummel J, Lisec J, Inostroza AC, Catchpole G, Willmitzer L. High-resolution direct infusion-based mass spectrometry in combination with whole 13C metabolome isotope labeling allows unambiguous assignment of chemical sum formulas. Anal Chem. 2008;80:9417–9425. doi: 10.1021/ac8014627. [DOI] [PubMed] [Google Scholar]
- Cui Q, Lewis IA, Hegeman AD, Anderson ME, Li J, Schulte CF, Westler WM, Eghbalnia HR, Sussman MR, Markley JL. Metabolite identification via the Madison Metabolomics Consortium Database. Nat Biotechnol. 2008;26:162–164. doi: 10.1038/nbt0208-162. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Steinfath M, Lisec J, Becher M, Witucka-Wall H, Torjek O, Fiehn O, Eckardt A, Willmitzer L, Selbig J, Altmann T. The metabolic signature related to high plant growth rate in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:4759–4764. doi: 10.1073/pnas.0609709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Meyer RC, Steinfath M, Redestig H, Becher M, Witucka-Wall H, Fiehn O, Torjek O, Selbig J, Altmann T, Willmitzer L. Identification of metabolic and biomass QTL in Arabidopsis thaliana in a parallel analysis of RIL and IL populations. Plant J. 2008;53:960–972. doi: 10.1111/j.1365-313X.2007.03383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MA, Caldana C, Steinhauser D, Balbo I, Fernie AR, Willmitzer L. Combined transcript and metabolite profiling of Arabidopsis grown under widely variant growth conditions facilitates the identification of novel metabolite-mediated regulation of gene expression. Plant Physiol. 2010;152:2120–2129. doi: 10.1104/pp.109.147306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman JM, Frederick RL, Sieburth LE. BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr Biol. 2004;14:1739–1746. doi: 10.1016/j.cub.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Van Norman JM, Sieburth LE. Dissecting the biosynthetic pathway for the bypass1 root-derived signal. Plant J. 2007;49:619–628. doi: 10.1111/j.1365-313X.2006.02982.x. [DOI] [PubMed] [Google Scholar]
- Kang YW, Kim RN, Cho HS, Kim WT, Choi D, Pai HS. Silencing of a BYPASS1 homolog results in root-independent pleiotrophic developmental defects in Nicotiana benthamiana. Plant Mol Biol. 2008;68:423–437. doi: 10.1007/s11103-008-9384-7. [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006;48:687–698. doi: 10.1111/j.1365-313X.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, Leyser O. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003;17:1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Turnbull CG, Murfet IC, Beveridge CA. Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol. 2001;126:1205–1213. doi: 10.1104/pp.126.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HM. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Davies W, Zhang J. Root Signals and the Regulation of Growht and Development of Plants in Drying Soil. Annual Rev Plant Physiol Plant mol Biol. 1991;42:55–76. doi: 10.1146/annurev.pp.42.060191.000415. [DOI] [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR. Effect of soil compaction on barley (Hordeum vulgare L.) growth 1. Possible role for ABA as a root-sourced chemical signal. Journal of Experimental Botany. 1996;47:539–549. doi: 10.1093/jxb/47.4.539. [DOI] [Google Scholar]
- Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Bari R, Datt Pant B, Stitt M, Scheible WR. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG. The role of long-distance signalling in plant responses to nitrate and other nutrients. J Exp Bot. 2002;53:39–43. doi: 10.1093/jexbot/53.366.39. [DOI] [PubMed] [Google Scholar]
- Tong H, Leasure CD, Hou X, Yuen G, Briggs W, He ZH. Role of root UV-B sensing in Arabidopsis early seedling development. Proc Natl Acad Sci USA. 2008;105:21039–21044. doi: 10.1073/pnas.0809942106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen MD, Naur P, Halkier BA. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004;37:770–777. doi: 10.1111/j.1365-313X.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D, May MJ, Sung ZR. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JC, Seeley KA, Sung ZR. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mahonen AP, Hauser MT, Helariutta Y. APL regulates vascular tissue identity in Arabidopsis. Nature. 2003;426:181–186. doi: 10.1038/nature02100. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;121:53–62. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- Scheres B, DiLaurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN. Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the radial axis. Development. 1995;121:53–62. [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/S0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/S0092-8674(00)80865-X. [DOI] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Dengler N. Cell cycling frequency and expression of the homeobox gene ATHB-8 during leaf vein development in Arabidopsis. Planta. 2002;216:212–219. doi: 10.1007/s00425-002-0847-9. [DOI] [PubMed] [Google Scholar]
- Beeckman T, Burssens S, Inze D. The peri-cell-cycle in Arabidopsis. J Exp Bot. 2001;52:403–411. doi: 10.1093/jexbot/52.suppl_1.403. [DOI] [PubMed] [Google Scholar]
- Dong X, Mindrinos M, Davis KR, Ausubel FM. Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991;3:61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2000;44:321–334. doi: 10.1023/A:1026592509060. [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano D, Fernandez-Busquets X, Schaller H, Gonzalez V, Boronat A, Arro M, Ferrer A. The metabolic imbalance underlying lesion formation in Arabidopsis thaliana overexpressing farnesyl diphosphate synthase (isoform 1S) leads to oxidative stress and is triggered by the developmental decline of endogenous HMGR activity. Planta. 2004;219:982–992. doi: 10.1007/s00425-004-1301-y. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Kanematsu S, Asada K. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 1997;38:1118–1126. doi: 10.1093/oxfordjournals.pcp.a029096. [DOI] [PubMed] [Google Scholar]
- Whetten RW, MacKay JJ, Sederoff RR. Recent Advances in Understanding Lignin Biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:585–609. doi: 10.1146/annurev.arplant.49.1.585. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lenk A, Andersson MX, Gjetting T, Pedersen C, Nielsen ME, Newman MA, Hou BH, Somerville SC, Thordal-Christensen H. A lesion-mimic syntaxin double mutant in Arabidopsis reveals novel complexity of pathogen defense signaling. Mol Plant. 2008;1:510–527. doi: 10.1093/mp/ssn011. [DOI] [PubMed] [Google Scholar]
- Smolen G, Bender J. Arabidopsis cytochrome P450 cyp83B1 mutations activate the tryptophan biosynthetic pathway. Genetics. 2002;160:323–332. doi: 10.1093/genetics/160.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmberg PL, Knox KA, Yun BW, Morris PC, Shafiei R, Hudson A, Loake GJ. The developmental selector AS1 is an evolutionarily conserved regulator of the plant immune response. Proc Natl Acad Sci USA. 2007;104:18795–18800. doi: 10.1073/pnas.0705586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003;132:1260–1271. doi: 10.1104/pp.103.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003;132:578–596. doi: 10.1104/pp.103.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyholos MK, Cavaness GF, Hall B, King E, Punwani J, Van Norman J, Sieburth LE. VARICOSE, a WD-domain protein, is required for leaf blade development. Development. 2003;130:6577–6588. doi: 10.1242/dev.00909. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant Journal. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Ahn IP, Kim S, Lee YH, Suh SC. Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol. 2007;143:838–848. doi: 10.1104/pp.106.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Hammes E, Plieth C, Desel C, Sattelmacher B, Hansen U-P. Effect of CO2 supply on formation of reactive oxygen species in Arabidopsis thaliana. Protoplasma. 2005;227:3–9. doi: 10.1007/s00709-005-0133-3. [DOI] [PubMed] [Google Scholar]
- Giraud E, Ho LH, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, Whelan J. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. doi: 10.1104/pp.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]