Abstract
Mercury is a major threat to the environment and to human health. It is highly desirable to develop a user-friendly kit for on-site mercury detection. Such a method must be able to detect mercury below the threshold levels for drinking water, 1–2 ppb. We developed a fluorescence method based on the oxymercuration of vinyl ethers to detect mercury in dental and environmental samples. Chloride ions interfered with the oxymercuration reaction, but the addition of AgNO3 solved this problem. Fine electronic and structural tuning led to the development of a more responsive probe that was less sensitive to chloride ion interference. This second generation probe could detect 1 ppb mercury ions in water.
Keywords: mercury, fluorescence, chemodosimeter, sensor, fluorescein, oxymercuration, vinyl ether, chloride
Introduction
Mercury is one of the most toxic metals, as seen in such unfortunate incidents as Minamata disease1 and mercury poisoning in Iraq.2 As these cases showed, methylmercury is extremely toxic and damages the central nervous system.3 The tragedy of Professor Karen Wetterhahn, who was studying organic mercury compounds,4 led to a moratorium on the use of these highly toxic and skin-permeable organic mercury compounds at many American institutions, including ours.5 Inorganic mercury compounds exhibit severe effects on the human heart, kidney, stomach, and genes.6 Despite the indisputable toxicity of mercury, it remains unclear whether there is a direct link between mercury and autism.7 Nonetheless, pregnant women are advised to avoid mercury-rich diets such as shark and tuna as a precaution.8
Mercury metal is volatile and can travel far in the air. It is then deposited on land or in water and oxidized to mercury(II).9 Oxidized inorganic mercury(II) may be converted to organic mercury by microorganisms.10 Mercury in the environment originates not only from geological events (e.g., volcano emissions11) but also from modern human activities. For example, the wastewater from dental offices is a major source of mercury pollution partly because dentists are exempt from federal regulations on waste.12 Coal-fired power plants generate substantial amounts of mercury, which is monitored by the United States Environmental Protection Agency (US EPA).13 In addition, the combination of human activities and geological samples such as the recent oil spill in the Gulf of Mexico raises great concern among the general public.14
Mercury is generally quantified by cold vapor atomic absorption spectroscopy15 or inductively coupled plasma mass spectroscopy.16 Although these methods are quantitative and powerful, the analyses require large and expensive instruments, highly trained personnel, and tedious maintenance. Mostly because of the size of the instruments, mercury-contaminated samples are generally analyzed off site. The off-site analysis format retards the remediation of wastewater17 in power plants and makes it essentially impractical to monitor dental offices and their surroundings.12 It is also important to note that these expensive and sophisticated analytical techniques cannot be employed in developing countries in which mercury pollution is severe and can be found even in tap water.18
Optical methods are more amenable to the on-site analysis of mercury with fewer resources. Therefore, numerous fluorescent chemosensors and chemodosimeters have been reported in the literature.19–22 These methods may facilitate mercury analyses, even if they are only semiquantitative. Because mercury is a soft Lewis acid, a vast majority of the chemosensors and chemodosimeters for mercury contain sulfur atom(s) that can tightly coordinate the metal as part of off-on fluorescence switches.19,21 Some of the chemosensors and chemodosimeters were successfully applied to real world “dirty” samples and in biological settings with low concentrations of mercury ions.19,22
As the protocol of the US EPA indicates,23 mercury-containing environmental samples are pretreated with harsh oxidants such as Cl-Br23 and H2O224 to transform various forms of organic and sulfur-bound (e.g., cysteine-bound) mercury species25 to sulfur-free inorganic mercury(II). Therefore, we felt that chemodosimeters and chemosensors for mercury in environmental and biological fluid samples might need to be compatible with oxidants. Although others have successfully demonstrated the utility of their sulfur-based indicators, we turned our attention to the π-electrophilicity of mercury ions and developed a chemodosimeter based on the oxymercuration of an alkyne.26 This chemodosimeter for mercury ions was found to be resistant to strong oxidants such as H2O2 and N-chlorosuccinimide (NCS). This method enabled us to detect mercury from fish and dental samples. However, the oxymercuration reaction needed to be heated to 90 °C. Moreover, we were not able to detect mercury ions below 4 ppb. The ability to detect mercury ions below this level is critically important because the limit of mercury concentration in drinking water is 2 ppb in the United States.27 We hypothesized that a more electron- rich π bond might be more reactive toward mercury ions, allowing for mercury detection at a lower temperature and at lower mercury concentrations. Herein, we report user-friendly and more sensitive new fluorescent chemodosimeters that react with mercury ions at an ambient temperature. The second-generation chemodosimeter in this study was found to be particularly powerful, detecting mercury ions at a 1 ppb level.
Results and Discussion
Design and synthesis of probe 3
An allyl ether is used as a protecting group and can be removed via a base-catalyzed olefin migration followed by either an acid-catalyzed hydrolysis at elevated temperature28 or mercury-promoted hydrolysis (Scheme 1a).29 Because the latter can be employed at an ambient temperature, we hypothesized that this transformation might be used as a means to selectively convert a nonfluorescent molecule to a fluorescent molecule with mercury ions.
Scheme 1.
(a) Two-step sequence to cleave an allyl ether. (b) Platform for a fluorescence off-on switch. (c) Preparation of 3 and its reaction with HgCl2 to form 1.
We previously demonstrated the conversion shown in Scheme 1b as a general platform for the development of fluorescence methods.26,30,31 In order to couple this platform with the chemistry shown in Scheme 1a, allyl ether 230,32,33 was treated with KOtBu in DMSO34 to form vinyl ether 3 in 86% yield as an inseparable mixture of cis and trans isomers (Scheme 1c). The purity of 3 was ensured by HPLC analysis (Figure S2, Supporting Information). The absorption and emission spectra of 3 are shown in Figure S1 (Supporting Information).
Reactivity of probe 3 with metal ions
Treatment of 3 with HgCl2 (1.0 equiv) at 25 °C afforded 1 in 82% isolated yield, indicating that this transformation could be used to develop a fluorescence method for Hg(II) (possibly HgCl+ because HgCl2 is mostly dissociated into HgCl+ and Cl-)35 at the more convenient temperature. Although vinyl ethers can be hydrolyzed under acidic conditions in a refluxing acetone-water mixture,28 such hydrolysis could not be detected when vinyl ether 3 was incubated in a pH 4 buffer at 80 °C, ensuring its stability during storage.
Next, we optimized conditions for a Hg(II)-promoted hydrolysis of vinyl ether 3 to form 133 in various buffers (for details, see Table S1, Supporting Information) at 25 °C. The ratio of Hg(II)-promoted and Hg(II)-free hydrolysis — the latter was negligible — was optimal at pH 3 (Figure 1a). However, the pH 3 reaction media was more difficult to neutralize than the pH 4 media for fluorescence measurement in a high throughput manner. Therefore, pH 4 was chosen for the remaining part of this study.
Figure 1.
Conversion of 3 to 1 at 25 °C. [3] = 1.0 μM for all of the experiments. Except for (c), all of the reactions were performed for 1 h. Fluorescence intensities were measured after addition of 1.23 M phosphate pH 7 buffer. (a) pH-dependence. (b) Metal selectivity in a 50 mM phthalate pH 4 buffer. Metal reagents: AgNO3, AuCl3, BaCl2, CaCl2, CdCl2•2.5H2O, CoCl2, CrCl3, CuCl2•2H2O, FeCl3, HgCl2, KCl, LiCl, MgCl2, MnCl2•4H2O, NaCl, NiCl2, Pb(NO3)2, Pd(NO3)2, PtCl2, Rh(PPh3)3, RuCl3, and ZnCl2. (c) Time-course of the oxymercuration reaction. [Hg(II)] = 0.30 μM, 50 mM phthalate pH 4 buffer. (d) Correlation between fluorescence intensities and [Hg(II)] in the presence and absence of N-chlorosuccinimide (NCS). The experiments were performed in a 50 mM phthalate pH 4 buffer in triplicate. The graph shows the mean values and standard deviations.
In a pH 4 buffer, we subjected vinyl ether 3 to various metal ions at 5 μM, demonstrating that the conversion of 3 to 1 was most efficiently promoted by Hg(II) (Figure 1b and Table S2, Supporting Information). A slight signal increase was observed in the presence of Pt(II).
The conversion of 3 to 1 in the presence of Hg(II) (0.30 μM) in a pH 4 buffer showed ~40% completion after 1 h, and the reaction continued to proceed (Figure 1c). The one-hour duration was considered an optimal balance between sensitivity and convenience. The fluorescence signals generated by the conversion of 3 to 1 was [Hg(II)] dependent with a distinct linear signal beginning as low as 4 ppb (= 20 nM) Hg(II) (● in Figure 1d; Figure S3a and Table S3 in Supporting Information).
Oxidative pretreatment of environmental samples with Br-Cl is a standard procedure by the US EPA. We found that NCS could also oxidatively disrupt the Hg-S bond.26 Because NCS can react with olefins, we were concerned about the stability of vinyl ether 3 toward NCS. However, this probe was stable against NCS while remaining responsive to Hg(II) (○ in Figures 1d and Table S3 in Supporting Information). These data indicate that probe 3 could be used to detect Hg(II) in sulfur-containing samples in the presence of NCS.
Failed application of 3 to detect Hg and revision of reaction conditions
We proceeded to apply the above method for the detection of Hg(II) in actual “dirty” samples in order to evaluate the utility of the method. Known amounts of Hg(II) were spiked into river water that contained < 1 ppb total mercury.36 The resulting solutions, after adjusting the pH to 4, were treated with 3 in an attempt to convert 3 to 1 but to no avail (Figure 3a, left). This failure prompted us to revisit the generality of the method. Rather than testing each metal separately, we examined mixtures of HgCl2 (2.5 μM) with various inorganic reagents (25 μM). Figure 2a (white bars) shows that most reagents interfered with the conversion of 3 to 1. It appeared that this interference was not caused by metals but by chloride ions. We compared the effect of NaCl and NaNO3 to verify the effect of chloride ions. Treatment of compound 3 (1.0 μM) with HgCl2 (2.5 μM) and excess NaCl (1.0 mM) in a pH 4 buffer showed no fluorescence increase (Figure 2b). Under similar reaction conditions, we only observed the starting material 3 by 1H NMR spectroscopy (Figure S18, Supporting Information). In contrast, the presence of NaNO3 did not impact the conversion of 3 to 1. We concluded that chloride ions interfered with our fluorescence method. This may also account for the failure of our earlier river-water study and limit the applications of 3 in biological systems.
Figure 3.
Applications of probe 3 for the detection of mercury species in environmental and dental samples. All detection was performed after adjustment of pH of samples to pH 4. (a) Comparison of river water and commercial pH 4 buffer. [HgCl2] = 2.2 μM (440 ppb). In the absence of AgNO3, Hg(II) cannot be detected by the method (left). In the presence of AgNO3, Hg(II) can be detected (middle). (b) Mercury detection in river water. [HgCl2] = 0–256 ppb, [AgNO3] = 2.0 mM. (c) Mercury detection in river water in the presence of organic compound. [AgNO3] = 2.0 mM, [compound] = 100 ppb. (d) Dental samples. [NCS] = 500 μM.
Figure 2.
Compatibility of our method to detect Hg(II) in the presence of inorganic ions. These experiments were performed in pH 4 buffer. (a) Interference by inorganic materials. [HgCl2] = 2.5 μM, [reagent shown] = 25 μM. (b) Interference by NaCl but not by NaNO3.
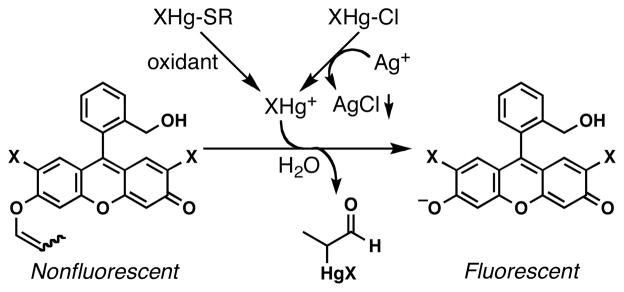
How did chloride ions interfere with the Hg(II)-promoted conversion of 3 to 1? The formation of 7 from the electrophilic species 5 (Scheme 1c) could be ruled out by the aforementioned NMR analysis. It is possible that the equilibrium shifted from more reactive HgX+ (X = Cl, phosphate, etc.) to less reactive HgXCl. This working hypothesis could account for the noninterference of the mixture of HgCl2 and AgNO3 (not shown). These results prompted us to hypothesize that the addition of excess AgNO3 to the mixtures of HgCl2 and MCln (M = Li, Na, etc.) might facilitate the conversion of HgCl2 (XHg-Cl bond: 24 kcal/mol37) to HgCl+ by virtue of the formation of poorly water-soluble AgCl38 (Ag-Cl bond: 71.7 kcal/mol37). In effect, the combination of HgCl2 (2.5 μM), AgNO3 (100 μM), and MCln (25 μM) generated a nearly uniform fluorescence signal (Figure 2a, gray bars). Thus, the addition of AgNO3 was found to afford a more general fluorometric detection method for Hg(II) in the presence of various inorganic molecules.
Detection of Hg(II) in river water
With this improvement, we attempted to detect mercury species in environmental samples again. In wastewater, the permitted discharge limits for total mercury may be 5 ppb39 or 10 ppb.40 Thus, as a proof-of-concept experiment,41 we proceeded to apply our method, which involved the use of AgNO3 for the detection of spiked Hg(II) (0–256 ppb) in river water (Figures 3a and 3b). Figure 3a shows that unlike the previously failed case (left), the addition of AgNO3 improves the signal recovery to nearly 100%. The standard curve in Figure 3b (also Table S4, Supporting Information) indicates that compound 3 could detect Hg(II) at ~8 ppb in river water.
We proceeded to determine whether the method based on the oxymercuration of vinyl ether 3 could be compatible with organic contaminants in environmental samples. We applied our method in the presence of various typical organic contaminants in wastewater42 to assess the robustness of 3 with other functional groups (Figure 3c and Table S5, Supporting Information). It was found that this probe could detect Hg(II) even if the reaction solution was contaminated with organic compounds, including an alkene.43 This may not be surprising because the olefin of compound 3 is more electron rich and thus more reactive toward Hg(II) than most alkenes.
Detection of Hg(II) in dental samples
In addition to environmental samples, dental samples were examined to broaden the applications of the fluorescence method. We hypothesized that cysteine from food might facilitate the dissolution of Hg from amalgam-filled teeth. Thus, the previously used two teeth26 were stirred in a cysteine solution. After the teeth were removed, the resulting solution was treated with NCS to oxidize the thiol and Hg-bound sulfur atoms before the addition of 3. Figure 3d and Table S6 (Supporting Information) show that we were able to detect, although unable to quantify,44 leached mercury from dental samples. The difference between bars 2 and 3 (3.63 × 104 in the raw data; see page S17 in the Supporting Information) was greater than three times the standard deviation of bar 2 (6.85 × 103), enabling us to detect Hg(II) with a confidence level of over 90%.44 Additional studies of dental samples are needed to validate the utility of the method in dentistry. Nonetheless, this result may warrant further studies on how sulfur-rich food may dissolve mercury amalgams.
It should be noted that, during this work, Ahn et al. published a very similar compound for Hg detection.45 There are notable differences: 3 cannot be contaminated with heavy metals, it reacts with Hg(II) in a 1:1 stoichiometry, and we discovered anion interference and a solution to this interference.
Design, preparation, and characterization of 12 and 13
While developing a protocol to circumvent interference by chloride ions, we realized that the chlorine atoms that were built into 3 might interfere, although organic and inorganic chlorides are not the same. Further consideration on removing the chlorides from 3 suggested that the vinyl ether of 12 (Scheme 2) might be more reactive toward Hg(II) due to the lack of electron-withdrawing chloride groups. Our minor concern was related to the inferiority of fluorescein over 2′,7′-dichlorofluorescein due to the higher pKa (6.4) of fluorescein compared to that of 2′,7′-dichlorofluorescein (4.3; pKa of compound 1 is 4.3).33 Nonetheless, we proceeded to synthesize compound 12 as shown in Scheme 2. This time, the olefin migration of the allyl ether 11 was highly stereoselective, only giving the cis product 12. Compound 12 also reacted with HgCl2 smoothly at 25 °C and gave the new fluorescent compound 13 in 72% isolated yield. The linear correlation was confirmed between the concentration of 13 and the fluorescence signals (Figure S4, Supporting Information).
Scheme 2.
Preparation of 12 and its reaction with HgCl2 to form 13.
The pKa of the phenolic hydroxy group of compound 13 was 6.0 (Figure 4a and Figure S5, Supporting Information), noticeably lower than that of fluorescein (6.4). This was unexpected because the conversion of the stronger electron-withdrawing carboxy group of fluorescein to the weaker hydroxymethyl group should decrease the acidity of the phenol hydroxy group. We postulate that the carboxylate anion of fluorescein might destabilize the phenoxy anion intramolecularly. Although the reason is not yet clear, the fine tuning of the acidity of phenol in fluorescein derivatives is a fruitful endeavor to develop new assay methods.46
Figure 4.
(a) pH titration of compound 13. Fluorescence intensity at 515 nm was monitored at ~0.3 pH intervals. (b) UV-Vis absorption spectra of 12 and 13 in 1% DMSO/pH 8 buffer. (c) Emission spectra of 12 and 13 in 0.1% DMSO/pH 8 buffer.
The UV-Vis absorption spectra of 12 and 13 were obtained as shown in Figure 4b. Compound 12 showed no absorption peak at ~490 nm but compound 13 did. The fluorescence emission spectra of 12 and 13 indicated that the signal of 12 was 140 times lower than that of 13 in a pH 8 buffer at 515 nm (λmax) at the same concentration (Figure 4c; see also Figure S1). This is consistent with the platform depicted in Scheme 1b.
Reactivity of 12 with metal ions
Solutions of the vinyl ether 12 were treated with Hg(II) (0.3 μM = 60 ppb) in pH 4, 5, 6, an 7 buffer (Figure 5a). Similarly to compound 3, compound 12 was more reactive at a lower pH. The reactivity of 12 toward Hg(II) was very high (Figure 5b). The only other metal that reacted with 12 was Pd(II) (cf. Figure 1b). In the presence of AgNO3, none of the coexisting metal reagents interfered with the Hg(II)-promoted conversion of 12 to 13 (Figure 5c). It was found that the fluorescence method using 12 allowed for the detection of 1 ppb Hg(II) (Figure 5d and Table S7, Supporting Information). Figure 5e indicates that chloride, bromide, and iodide ions interfered with the Hg(II)-promoted conversion of 12 to 13, but nitrate and sulfate ions did not. The interference by chloride ions was less severe for compound 12 than for compound 3 (Figure S6, Supporting Information). The halide-ion-mediated interference could be overcome by the addition of AgNO3 (Figure 5c).
Figure 5.
Comparison of reaction conditions for the conversion of 12 to 13. (a) pH-dependence. Fluorescence intensities were measured after addition of 1.3 M pH 7 buffer and 500 mM pH 10 buffer. (b) Metal selectivity. All the metals were tested at 5 μM in pH 4 buffer. (c) Hg detection in the presence of various metal ions. All of the reactions were performed in pH 4 buffer. Metal reagents: AgNO3, AuCl3, BaCl2, CaCl2, CdCl2•2.5H2O, CoCl2, CrCl3, CuCl2•2H2O, FeCl3, HgCl2, KCl, LiCl, MgCl2, MnCl2•4H2O, NaCl, NiCl2, Pb(NO3)2, Pd(NO3)2, PtCl2, Rh(PPh3)3, RuCl3, and ZnCl2. (d) Correlation between fluorescence intensities at 515 nm and [Hg(II)]. The experiments were performed at pH 4 in triplicate. (e) The effect of anion. All detection was performed in pH 4 buffer.
Next, we compared the new chemodosimeter 12 with 3 and 14.26 As Figure 6a shows, compound 12 was far more reactive than 14 toward Hg(II) in a pH 4 buffer at 25 °C. For example, after 10 min the fluorescence increase with 12 was 242 times greater than that with 14. The reaction with 12 was >60% complete after 1 h. It is even more noteworthy that the initial rate was about 12 times greater for 12 relative to 3 (Figure 6b). Additionally, compound 12 was more reactive than compound 3 at pH 7 (Figure 6c), implying potential biological applications.
Figure 6.
Comparisons of reactivity between probes developed by our group. [probe] = 1.0 μM, [Hg(II)] = 0.3 μM. (a) Time-course of the oxymercuration reaction at 25 °C in 50 mM phthalate pH 4.0 buffer. The comparison between probe 12 and 14 was shown. (b) Time-course of the oxymercuration reaction at 25 °C in 50 mM phthalate pH 4.0 buffer. The comparison between probe 3 and 12 was shown. To focus on their initial rate, fluorescence measurements were carried out every 1 min for 18 min. (c) Time-course of the oxymercuration reaction in 50 mM phosphate pH 7 buffer. The comparison between probe 3 and 12 was shown.
Application with river water
We applied chemodosimeter 12 for detection of Hg(II) spiked in river water again. Chemodosimeter 12 was able to detect 1 ppb Hg in river water (Figure 7 and Table S8, Supporting Information), which is below the US EPA’s limitation for drinking water (2 ppb). The heightened sensitivity of 12 in determining [Hg(II)] in such a complex media as natural water indicates its robustness and potential for facile on-site monitoring of water safety. When our method was applied to the analysis of two wastewater samples from a coal-fired power plant, we found compound 12 to be a qualitative indicator of Hg(II) (Supporting Information). Although we were unable to quantitatively assess Hg(II) concentrations in these more complex samples, we were able to qualitatively discriminate between concentration values as low as 2 ppb and <500 ppt. Further studies are needed to determine the scope and limitations of the use of 12 with additional complex samples.
Figure 7.

Application of 12 for the detection of Hg(II) in river water. Titration of Hg(II) in river water was performed. [12] = 1.0 μM; [Hg(II)] = 0, 0.25, 0.5, 1, 2, and 4 ppb; [AgNO3] = 2.0 mM; 0.5% DMSO in 50 mM phthalate pH 4 water; 1 h; 25 °C.
Conclusions
In summary, we have developed a sensitive and selective fluorometric method to detect mercury species at an ambient temperature in the presence of various organic, inorganic, and anionic contaminants. The method with 3 was effective in the detection of Hg(II) in river water and dental samples. Further structural fine-tuning led to the development of the vinyl ether 12 (Scheme 3). Compound 12 could react with Hg(II) after the removal of chloride ions with AgNO3. This compound was 242 times more reactive than the previously reported alkyne 14 toward Hg(II) at an ambient temperature and could be used to detect 1 ppb Hg(II). Further evaluations of this method with additional real-world samples are underway in our laboratory.
Scheme 3.
Summary of this work.
Experimental Section
Synthetic Materials and Methods
All of the reactions in Scheme 1c and 2 were carried out with commercial-grade reagents without further purification. DMF was used after distillation from silica gel. CH2Cl2 was used after distillation from CaH2. Yields refer to chromatographically and spectroscopically (1H NMR) homogeneous materials. All reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-254) using UV light (254 nm) for visualization or phosphomolybdic acid in ethanol as developing agents and heat for visualization. TSI silica gel (230–400 mesh) was used for flash chromatography. NMR spectra were recorded on AM300 or AM400 (Bruker) instruments and calibrated using a solvent peak as an internal reference. The following abbreviations are used to indicate the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, app = apparent. High-resolution mass spectra were obtained using EBE geometry.
Preparation of Compound 3
KOtBu (11 mg, 0.10 mmol) was added to a solution of compound 230 (21 mg, 50 μmol) in DMSO (1.0 mL) at 25 °C under a nitrogen atmosphere, and the resulting solution was heated in a 90 °C oil bath for 12 h. The reaction mixture was then poured onto ice-cold H2O (5.0 mL), and the resulting mixture was extracted with EtOAc (2 × 5 mL). The combined organic layers were washed with brine (10 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash column chromatography (5% to 20% EtOAc in hexanes) on silica gel (10 mL) to afford compound 3 (18 mg, 86%, mixture of cis/trans (2:1)) as an orange solid. Data for 3: m.p. = 146–155 °C; Rf = 0.31 (30% EtOAc in hexanes); IR (KBr pellet): νmax = 3368 (broad, O-H), 2921, 2859, 1606 (C=O), 1482, 1435, 1411, 1269, 1175, 1034, 874, 725 cm−1; 1H NMR (300 MHz, acetone-d6, 293 K, Figure S7): δ = 7.52–7.39 (m, 2H, Ar), 7.34–7.27 (m, 1H, Ar), 7.05 (s, 0.67H, Ar (cis isomer)), 7.03 (s, 0.33H, Ar (trans isomer)), 6.99–6.97 (m, 2H, Ar), 6.91–6.88 (m, 1H, Ar), 6.87 (s, 1H, Ar), 6.68 (dq, J = 12.0, 1.8 Hz, 0.33H, CH3CH=CHOAr (trans isomer)), 6.65 (dq, J = 6.6 (A similar coupling constant was observed in a related compound47), 1.8 Hz, 0.67H, CH3CH=CHOAr (cis isomer)), 5.51 (dq, J = 12.0, 6.6 Hz, 0.33H, CH3CH=CHOAr (trans isomer)), 5.42 (s, 2H, ArCH2OH), 5.13 (dq, J = 6.6,47 6.6 Hz, 0.67H, CH3CH=CHOAr (cis isomer)), 1.76–1.66 (m, 3H, CH3CH=CHOAr) ppm; 13C NMR (75 MHz, acetone-d6, 293 K, Figure S8): δ = 154.6, 154.2, 154.0, 150.44, 150.38, 145.8, 142.1, 140.9, 139.7, 130.7, 130.4, 129.5, 124.1, 122.3, 121.0, 118.8, 118.4, 118.3, 117.2, 111.7, 110.7, 104.7, 104.5, 104.2, 83.6, 73.4, 12.3, 9.6 ppm; HRMS (EI+) m/z calcd. for C23H16O4Cl2 [M]+ 426.0426, found 426.0425.
Conversion of compound 3 to compound 1
HgCl2 (14 mg, 50 μmol) was added to a solution of compound 3 (21 mg, 50 μmol) in a mixture of 1:9 DMSO/50 mM phthalate pH 4 buffer (20 mL). The resulting mixture was stirred at 25 °C for 1 h and extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with brine (2 × 30 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash column chromatography (10% to 70% EtOAc in hexanes) on silica gel (10 mL) to afford compound 1 (16 mg, 82%) as an orange solid. The spectroscopic data of compound 1 were consistent with the literature.30 Compound 8 was isolated from other fractions. Rf = 0.66 (50% EtOAc in hexanes); 1H NMR (300 MHz, acetone-d6, 293 K, Figure S9): δ = 9.63 (d, J = 1.2 Hz, 1H, CHO), 3.66 (qd, J = 6.9, 1.2 Hz, 1H, OHC–CHHgX), 1.53 (d, J = 6.9 Hz, 3H, CH3) ppm. Due to the extreme toxicity of organomercury species, we discarded this material in collaboration with the Environmental Health and Safety office at the University of Pittsburgh (http://www.ehs.pitt.edu) without obtaining further spectroscopic data.
Preparation of Compound 10
K2CO3 (4.15 g, 30.0 mmol) was added to a solution of fluorescein (3.32 g, 10.0 mmol) in DMF (20 mL) at 25 °C under a nitrogen atmosphere, followed by allyl bromide (2.60 mL, 30 mmol). After stirring for 48 h at 25 °C, the reaction mixture was poured onto H2O (500 mL). The resulting mixture was then extracted with EtOAc (3 × 200 mL), and the combined extracts were washed with H2O (3 × 500 mL) and brine (500 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was recrystallized from hexanes-EtOAc to afford compound 10 (3.17 g, 77%) as an orange solid. Data for 10: m.p. = 153–155 °C; Rf = 0.34 (60% EtOAc in hexanes); IR (KBr pellet): νmax = 3054, 2986, 2932, 1727 (C=O), 1643 (C=O), 1595, 1517, 1481, 1256, 1211, 1106, 855, 759 cm−1; 1H NMR (300 MHz, CDCl3, 293 K, Figure S10): δ = 8.27 (dd, J = 7.5, 1.5 Hz, 1H, Ar), 7.75 (ddd, J = 7.5, 7.5, 1.5 Hz, 1H, Ar), 7.68 (ddd, J = 7.5, 7.5, 1.5 Hz, 1H, Ar), 7.32 (dd, J = 7.5, 1.5 Hz, 1H, Ar), 6.96 (d, J = 2.4 Hz, 1H, Ar), 6.90 (d, J = 9.0 Hz, 1H, Ar), 6.87 (d, J = 9.6 Hz, 1H, Ar), 6.77 (dd, J = 9.0, 2.4 Hz, 1H, Ar), 6.56 (dd, J = 9.6, 1.8 Hz, 1H, Ar), 6.47 (d, J = 1.8 Hz, 1H, Ar), 6.07 (dddd, , J = 17.4, 10.5, 5.4, 5.4 Hz, 1H, CH2=CHCH2OAr), 5.60 (dddd, , J = 17.4, 10.2, 6.0, 6.0 Hz, 1H, ArCO2CH2CH=CH2), 5.46 (dd, J = 17.4, 1.2 Hz, 1H, HtransCH=CHCH2OAr), 5.37 (dd, J = 10.5, 1.2 Hz, 1H, HCHcis=CHCH2OAr), 5.14–5.08 (m, 2H, ArCO2CH2CH=CH2), 4.66 (ddd, J = 5.4, 1.2, 1.2 Hz, 2H, CH2=CHCH2OAr), 4.48 (dddd, J = 12.9, 6.0, 1.2, 1.2 Hz, 1H, ArCO2CHaHCH=CH2), 4.46 (dddd, J = 12.9, 6.0, 1.2, 1.2 Hz, 1H, ArCO2CHHbCH=CH2) ppm; 13C NMR (75 MHz, CDCl3, 293 K, Figure S11): δ = 185.8, 165.2, 163.1, 159.1, 154.3, 150.2, 134.5, 132.9, 132.0, 131.4, 131.1, 130.67, 130.65 130.4, 130.1, 129.9, 129.1, 119.3, 118.9, 117.9, 115.1, 113.9, 105.9, 101.3, 69.6, 66.2 ppm; HRMS (ESI+) m/z calcd. for C26H21O5 [M]+ 413.1389, found 413.1373.
Preparation of Compound 11
A solution of DIBALH (9.6 mL, 1.0 M in CH2Cl2) was added dropwise to a solution of compound 10 (0.83 g, 2.0 mmol) in CH2Cl2 (7.0 mL) over 15 min at −78 °C under a nitrogen atmosphere. The resulting solution was stirred at the same temperature for 10 min and then warmed to 25 °C. After stirring at the same temperature for 2 h, Et2O (5.0 mL) was added to the resulting solution at 0 °C with stirring, and then saturated aqueous NH4Cl (3.5 mL) was added dropwise to the mixture at the same temperature. This mixture was warmed to 25 °C again and stirred for 1 h. The reaction mixture was then diluted with Et2O (5.0 mL), and DDQ (0.45 g, 2.2 mmol) was added slowly to this mixture at 0 °C. After being stirred for 1 h at 25 °C, the mixture was filtered through a pad of Celite®, and the pad was rinsed with EtOAc. The filtrate was dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash column chromatography (10 to 30 % EtOAc in hexanes) on silica gel (180 mL) to afford compound 11 (701 mg, 98%) as a pale yellow solid. Data for 11: m.p. = 144–146 °C; Rf = 0.32 (30% EtOAc in hexanes); IR (KBr pellet): νmax = 3228 (broad, OH), 2921, 2852, 1616 (C=O), 1501, 1517, 1456, 1430, 1335, 1224, 1180, 1003, 841, 759 cm−1; 1H NMR (300 MHz, acetone-d6, 293 K, Figure S12): δ = 7.44 (d, J = 7.5 Hz, 1H, Ar), 7.37 (dd, J = 7.5, 7.5 Hz, 1H, Ar), 7.26 (dd, J = 7.5, 7.5 Hz, 1H, Ar), 6.89 (d, J = 8.7 Hz, 1H, Ar), 6.84 (d, J = 7.5 Hz, 1H, Ar), 6.83 (d, J = 8.7 Hz, 1H, Ar), 6.76 (d, J = 2.4 Hz, 1H, Ar), 6.67 (d, J = 2.4 Hz, 1H, Ar), 6.66 (dd, J = 8.7, 2.4 Hz, 1H, Ar), 6.58 (dd, J = 8.7, 2.4 Hz, 1H, Ar), 6.07 (dddd, , J = 17.4, 10.5, 5.4, 5.4 Hz, 1H, CH2=CHCH2OAr), 5.42 (dd, J = 17.4, 1.2 Hz, 1H, HtransCH=CHCH2OAr), 5.28 (s, 2H, ArCH2OH), 5.24 (dd, J = 10.5, 1.2 Hz, 1H, HCHcis=CHCH2OAr), 4.61 (ddd, J = 5.4, 1.2, 1.2 Hz, 2H, CH2=CHCH2OAr) ppm; 13C NMR (75 MHz, acetone-d6, 293 K, Figure S13): δ = 160.2, 159.0, 152.2, 152.1, 146.7, 140.1, 134.5, 131.0, 130.9, 129.1, 128.8, 124.3, 121.9, 118.9, 117.74, 117.67, 112.7, 112.3, 107.8, 101.9, 84.1, 72.7, 69.5 ppm; HRMS (ESI+) m/z calcd. for C23H18O4Na [M+Na]+ 381.1103, found 381.1072.
Preparation of Compound 12
KOtBu (56 mg, 0.44 mmol) was added to a solution of compound 11 (72 mg, 0.20 mmol) in DMSO (1.7 mL) at 25 °C under a nitrogen atmosphere, and the resulting solution was heated in a 50 °C oil bath for 1 h. The reaction mixture was then poured onto ice-cold H2O (10 mL), and the resulting mixture was extracted with EtOAc (2 × 10 mL). The combined organic layers were washed with brine (30 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash column chromatography (10% to 30% EtOAc in hexanes) on silica gel (10 mL) to afford compound 12 (70 mg, 97%) as an orange solid. Data for 12: m.p. = 96–98 °C; Rf = 0.30 (40% EtOAc in hexanes); IR (KBr pellet): νmax = 3304 (broad, O-H), 3045, 2921, 2858, 1610 (C=O), 1497, 1459, 1426, 1270, 1177, 1109, 1023, 846, 804, 725 cm−1; 1H NMR (400 MHz, acetone-d6, 293 K, Figure S14): δ = 7.47 (d, J = 7.6 Hz, 1H, Ar), 7.39 (dd, J = 7.6, 7.6 Hz, 1H, Ar), 7.28 (dd, J = 7.6, 7.6 Hz, 1H, Ar), 6.96 (d, J = 8.8 Hz, 1H, Ar), 6.84 (d, J = 8.8 Hz, 1H, Ar), 6.83 (d, 6.82 J = 7.6 Hz, 1H, Ar), (d, J = 2.4 Hz, 1H, Ar), 6.75 (dd, J = 8.8 2.4 Hz, 1H, Ar), 6.67 (d, J = 2.4 Hz, 1H, Ar), 6.60–6.55 (m, 2H, Ar and CH3CH=CHOAr), 5.30 (s, 2H, ArCH2OH), 4.96 (dq, J = 6.8, 6.8 Hz, 1H, CH3CH=CHOAr), 1.68 (dd, J = 6.8, 1.6 Hz, 3H, CH3CH=CHOAr) ppm; 13C NMR (100 MHz, acetone-d6, 293 K, Figure S15): δ = 159.2, 158.7, 152.12, 152.07, 146.7, 141.4, 140.0, 131.2, 131.0, 129.1, 128.9, 124.3, 121.9, 120.7, 117.6, 112.9, 112.7, 108.7, 103.5, 102.8, 84.0, 72.9, 9.6 ppm; HRMS (ESI+) m/z calcd. for C23H18O4Na [M+Na]+ 381.1103, found 381.1083.
Conversion of compound 12 to compound 13
HgCl2 (18 mg, 67 μmol) was added to a solution of compound 12 (24 mg, 67 μmol) in a mixture of 1:9 DMSO/pH 4 buffer (26 mL). The resulting mixture was stirred at 25 °C for 1 h and extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with brine (2 × 50 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash column chromatography (10% to 70% EtOAc in hexanes) on silica gel (10 mL) to afford compound 13 (15 mg, 72%) as an orange solid. Data for 13: m.p. = > 200 °C; Rf = 0.32 (50% EtOAc in hexanes); IR (KBr pellet): νmax = 3430 (broad, O-H), 2869, 2600, 1602 (C=O), 1455, 1379, 1309, 1241, 1207, 1117, 860, 757 cm−1; 1H NMR (300 MHz, acetone-d6, 293 K, Figure S16): δ = 7.45 (d, J = 7.5 Hz, 1H, Ar), 7.34 (dd, J = 7.5, 7.5 Hz, 1H, Ar), 7.27 (dd, J = 7.5 7.5 Hz, 1H, Ar), 6.83 (d, J = 7.5 Hz, 1H, Ar) 6.81 (d, J = 8.4 Hz, 2H, Ar), 6.64 (d, J = 2.4 Hz, 2H, Ar), 6.56 (dd, J = 8.4, 2.4 Hz, 2H, Ar), 5.26 (s, 2H, ArCH2OH) ppm; 13C NMR (75 MHz, acetone-d6, 293 K, Figure S17): δ = 159.0, 152.3, 146.9, 140.2, 131.0, 129.1, 128.8, 124.4, 121.8, 117.9, 112.6, 102.8, 84.2, 72.6 ppm; HRMS (EI+) m/z calcd. for C20H15O4 [M]+ 319.0970, found 319.0999.
Sample preparations
Mercury standard solution (20 ppm in 5% HNO3) was purchased from the RICCA Chemical Company (Arlington, Texas) and used as received. The other mercury standard solution (10,000 ppm in 2% HNO3) was purchased from Ultra Scientific (North Kingstown, Rhode Island) and used as received. OmniTrace Ultra™ High Purity Acid HNO3 (Hg < 10 ppt) was purchased from EMD (Item number NX0408, Lot number 48157) and used as received. ARISTAR® ULTRA water was purchased from VWR (Catalog number 7732-18-5) and used as received. AgNO3 was purchased from EMD and used as received. River water was collected from the Allegheny River on January 29, 2008. The pH 3 buffer solution was made from a commercial pH 4 buffer (50 mM) and a pure HNO3 solution.
Metal solutions (1.0 mM)
AuCl3, BaCl2, NiCl2, CrCl3, Pb(NO3)2, NaCl, MnCl2•4H2O, MgCl2, CoCl2, HgCl2, AgNO3, ZnCl2, LiCl, CuCl2•2H2O, and CaCl2 were dissolved in H2O. FeCl3, CdCl2•2.5H2O, KCl, Rh(PPh3)3, and RuCl3 were dissolved in MeOH. PtCl2 was dissolved in MeOH/acetone (1:1). Pd standard solution (High-Purity Standards, Cat. No. 100038-1) was diluted with 1% HNO3. The resulting solution was used as the Pd solution.
Fluorescence Measurement
All samples were incubated at 25 °C, and the pH values of the solutions were adjusted to an appropriate pH range (pH >5 for 1, pH >8 for 13) by the addition of 1.23 M phosphate pH 7 buffer (for 1, 4.0% of the total volume of a reaction solution) or a 1:5 mixture of 1.23 M phosphate pH 7 buffer and 500 mM borate pH 10 buffer (for 13, 24% of the total volume of reaction solution). The resulting samples were vortexed for 5 s prior to fluorescence measurement. Fluorescence spectra were recorded in a 1 × 1-cm disposable cuvette (VWR; catalog number 58017-880) on a Jobin Yvon FluoroMax-3 spectrometer under the control of a Windows-based PC running FluorEssence software. The samples were excited at 497 nm and the emission intensities were collected at 523 nm (for 1) or 515 nm (for 13). All spectra were corrected for emission intensity using the manufacturer-supplied photomultiplier curves.
The pH dependence of Hg detection (Figures 1a and 5a)
Reaction conditions: [Hg(II)] = 0.30 μM, [3 or 12] = 1.0 μM, 0.05% DMSO in buffer (5.0 mL), 25 °C, 1 h. Protocol: A 100 μM Hg standard solution (15 μL each) was added to a pH 3, 4, 5, 6, or 7 buffer (4.98 mL each). A 1.0 mM solution of 3 or 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to each of these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization. Note: For the pH 3 sample, an additional 1.0 mL of 500 mM borate pH 10 buffer was required to obtain a pH >5 solution.
Metal selectivity (Figures 1b and 5b)
Reaction conditions: [metal] = 5.0 μM, [3 or 12] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: Each of 1.0 mM solutions of metal reagents in appropriate solvents (25 μL, see Supporting Information for details) was added to a 50 mM phthalate pH 4 buffer (4.97 mL). A 1.0 mM solution of 3 or 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Time-course of the oxymercuration reaction (Figures 1c, 6a, 6b, and 6c)
Reaction conditions: [Hg(II)] = 0.30 μM, [3, 12, or 14] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer or a 50 mM pH 7 phosphate buffer (20 mL), 25 °C. Protocol: A 100 μM Hg standard solution (60 μL) was added to a 50 mM phthalate pH 4 buffer (Figures 1c, 6a and 6b) or a 50 mM phosphate pH 7 buffer (Figure 6c) (20 mL). A 1.0 mM solution of 3, 12, or 14 in 1:1 DMSO/50 mM phosphate pH 8 buffer (20 μL) was added to each of the solutions, and the resulting reaction solutions were shaken for 3 s and incubated at 25 °C before fluorescence measurement. A fraction (1.5 mL) of each of these reaction solutions was taken for the measurement at 10, 20, 30, 40, 50, 60, 90, 120, 240, 480, and 1140 min. For Figure 6b, this measurement was carried out every 1 min for 18 min with 1.0 mL of the reaction solution. See the “Fluorescence Measurement” section for sample neutralization.
Titration curve (Figures 1d and 5d)
Reaction conditions: [Hg(II)] = 0, 1, 2, 4, 8, 16, and 32 ppb, [NCS] = 10 μM for NCS(+) samples and 0 μM for NCS(−) samples, [3 or 12] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 0, 200, 400, 800, 1600, 3200, or 6400 ppb solution of HgCl2 in 5% HNO3 (25 μL) and a 2.0 mM solution of NCS in water (25 μL) were added to each of 50 mM phthalate pH 4 buffers (4.95 mL). A 1.0 mM solution of 3 or 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to each of these solutions, and the resulting solutions were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Hg detection in the presence of other metal (Figure 2a (white bar))
Reaction conditions: [Hg(II)] = 2.5 μM, [other metal] = 25 μM, [3] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 100 μM Hg standard solution in 5% HNO3 (125 μL) and each of the 1.0 mM metal solutions (125 μL; see Supporting Information for detail) were added to each of 50 mM phthalate pH 4 buffer (4.75 mL). Each of these solutions was treated with a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL). The resulting solutions were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
The effect of chloride or nitrate ion (Figures 2b and S6 (Supporting Information))
Reaction conditions: [Hg(II)] = 2.5 μM, [Cl− or NO3−] = 1.0 mM, [3 or 12] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 1.0 mM HgCl2 solution in 5% HNO3 (12.5 μL) and a 100 mM NaCl or NaNO3 solution in water (50 μL) were added to a 50 mM phthalate pH 4 buffer (4.93 mL). Each of these solutions was treated with a 1.0 mM solution of 3 or 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL). The resulting samples were shaken for 3 s and incubated at 25 °C for 15 min (Figure 2b) or 1 h (Figure 5c) before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
The interference of inorganic materials with AgNO3 (Figures 2a (gray bar) and 5c)
Reaction conditions for Figure 2a: [Hg(II)] = 2.5 μM, [other metal] = 25 μM, [AgNO3] = 100 μM, [3] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 1.0 mM HgCl2 solution in 5% HNO3 (12.5 μL) and a 1.0 mM solution of another metal (125 μL) were added to a 50 mM phthalate pH 4 buffer (4.86 mL). These solutions were vortexed for 5 s, and then a 10 mM solution of AgNO3 in ultra pure water (50 μL) was added to the resulting solutions. After vortexing for 5 s, a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Reaction conditions for Figure 5c: [Hg(II)] = 1.0 μM, [other metal] = 10 μM, [AgNO3] = 2.0 mM, [12] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 1.0 mM HgCl2 solution in 5% HNO3 (5.0 μL) and a 1.0 mM solution of another metal (50 μL) were added to a 50 mM phthalate pH 4 buffer (4.84 mL). These solutions were vortexed for 5 s, and then a 100 mM solution of AgNO3 in ultra pure water (100 μL) was added to the resulting solutions. After vortexing for 5 s, a 1.0 mM solution of 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Detection of Hg in river water by probe 3 (Figures 3a and 3b)
Reaction conditions for Figure 3a: [Hg(II)] = 440 ppb (550 ppb = 2.75 μM in river before dilution), [AgNO3] = 2.0 mM, [3] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.09 mL), 25 °C, 1 h. Protocol: River water (3.98 mL) was spiked with a 440 μM solution of HgCl2 in 5% HNO3 (25 μL). This solution was treated with a 250 mM phthalate pH 4 buffer (1.0 mL) and then a 100 mM solution of AgNO3 in ultra pure water (100 μL). After vortexing for 5 s, a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to this solution, and the resulting sample was shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Reaction conditions for Figure 3b: [Hg(II)] = 0, 1, 2, 4, 8, 16, 32, 64, 128, and 256 ppb, [AgNO3] = 2.0 mM, [3] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.1 mL), 25 °C, 1 h. Protocol: A 0, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8, 25.6, or 51.2 ppm solution of HgCl2 in 5% HNO3 (25 μL) was spiked into each of river water samples (4.0 mL each). Each of these solutions was treated with a 250mM phthalate pH 4 buffer (1.0 mL each) and then a 100 mM solution of AgNO3 in ultra pure water (100 μL each). After being shaken for 3 s, a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Hg detection in the presence of organic compound (Figure 3c)
Reaction conditions: ([Hg(II)] = 16 ppb, [organic contaminant] = 80 ppb, [AgNO3] = 2 mM, [3] = 1 μM, 1.3% DMSO in a 50 mM phthalate pH 4 buffer (1.30 mL), 25 °C, 1 h. Protocol: River water (1.0 mL) was spiked with a 10 μM HgCl2 solution in 5% HNO3 (10 μL) and a 10 ppm solution of an organic contaminant in DMSO (phenol, acetophenone, caffeine, or cholesterol, 10 μL). Each of these solutions contained 0.1 μM (20 ppb) Hg(II) and 100 ppb compound. Each of the resulting solutions was treated with a 250 mM phthalate pH 4 buffer (250 μL each) and then a 100 mM solution of AgNO3 in ultra pure water (25 μL). After vortexing for 5 s, a 0.1 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (12.5 μL) was added to this solution, and the resulting sample was shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Detection of Hg from dental amalgam (Figure 3d)
Reaction conditions: Two teeth filled with mercury amalgam, [cysteine] = 50 μM, [NCS] = 500 μM, [3] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (4.06 mL), 25 °C, 1 h. Protocol: Two teeth filled with an amalgam were added to a 20 mM solution of cysteine in water (2.0 mL), and the resulting mixture was shaken (200 rpm) for 1 h at 37 °C.26 A portion (5.0 μL) of the resulting solution was added to a 50 mM phthalate pH 4 buffer (2.0 mL). After being shaken for 3 s, a 20 mM solution of NCS in water (50 μL) and a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (2.0 μL) were added to the resulting solution. A negative control sample was prepared by adding two teeth filled with an amalgam into water (2.0 mL) and shaken (200 rpm) for 1 h at 37 °C. A portion (5.0 μL) of the resulting mixture was added to a 50 mM phthalate pH 4 buffer (2.0 mL). This mixture was then treated with a 20 mM solution of NCS in water (50 μL) and a 1.0 mM solution of 3 in 1:1 DMSO/50 mM phosphate pH 8 buffer (2.0 μL). The resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
The pH titration of compound 13 (Figure 4a)
An aqueous solution (100 mL) containing 13 (5 μM) and NaCl (1.0 M) was prepared. To one half of this mixture solution (50 mL) was added a small magnetic stir bar in a vial with a pH electrode. The pH of the solution was changed by adding 0.1 N or 1 N HCl solution dropwise while stirring and the fluorescence spectrum was recorded at ~0.3 pH intervals. The other half of this mixture solution (50 mL) was titrated with 0.1 N or 1 N NaOH solution. In order to calculate the pKa, the pH dependence of fluorescence spectra were analyzed using the following equation: pH = pKa-log[(Fmax-F)/(F-Fmin)].48
The effect of anion (Figure 5e)
Reaction conditions: [Hg(II)] = 0.5 μM, [Cl−, Br−, I−, SO42−, or NO3−] = 2.0 mM, [12] = 1.0 μM, 0.05% DMSO in a 50 mM phthalate pH 4 buffer (5.0 mL), 25 °C, 1 h. Protocol: A 0.5 mM HgCl2 solution in 5% HNO3 (5.0 μL) and a 100 mM solution of either NaCl, NaBr, NaI, Na2SO4, or NaNO3 in water (100 μL) were added to a 50 mM phthalate pH 4 buffer (4.90 mL). A 1.0 mM solution of 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (5.0 μL) was added to these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Detection of Hg spiked in river water by probe 12 (Figure 7)
Reaction conditions: [Hg(II)] = 0, 0.25, 0.5, 1, 2, and 4 ppb, [AgNO3] = 2.0 mM, [12] = 1.0 μM, 0.5% DMSO in a 50 mM phthalate pH 4 buffer (1.02 mL), 25 °C, 1 h. Protocol: A 50 mM phthalate pH 4 buffer (20 mL) was dried under reduced pressure to obtain a solid in a test tube. River water (20 mL) was passed through a syringe filter and poured into the test tube to adjust the pH to 4. A 0, 62.5, 125, 250, 500, or 1000 ppb solution of HgCl2 in 5% HNO3 (4 μL) was spiked into each of the resulting river water samples (1.0 mL each). A 100 mM solution of AgNO3 in ultra pure water (20 μL each) was added to each of these samples, and the resulting mixtures were vortexed for 5 s and centrifuged (1 min, 2000 rpm) to remove precipitates. A 0.1 mM solution of 12 in 1:1 DMSO/50 mM phosphate pH 8 buffer (10 μL) was added to each of these solutions, and the resulting samples were shaken for 3 s and incubated at 25 °C for 1 h before fluorescence measurement. See the “Fluorescence Measurement” section for sample neutralization.
Supplementary Material
Acknowledgments
We thank Nalco company for the wastewater samples. This work was supported by the US National Science Foundation (CHE-0616577 and CHE-0911092) and the National Institutes of Health (R01CA120792).
Footnotes
Supporting Information Available: Experimental procedures for all fluorescence analyses and chemodosimeter preparation. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Harada M. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]; (b) Weiss B. Toxicol Sci. 2007;97:223–225. doi: 10.1093/toxsci/kfm047. [DOI] [PubMed] [Google Scholar]
- 2.(a) Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Pediatrics. 1974;54:587–595. [PubMed] [Google Scholar]; (b) Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, Tikriti S, Dhahir HI, Clarkson TW, Smith JC, Doherty RA. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 3.Atchison WD, Hare MF. FASEB J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- 4.Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, Toribara TY, Cernichiari E, Clarkson T. N Engl J Med. 1998;338:1672–1676. doi: 10.1056/NEJM199806043382305. [DOI] [PubMed] [Google Scholar]
- 5.To comply with a moratorium at our institution, this work does not involve the deliberate use of organic mercury compounds.
- 6.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environ Toxicol. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 7.(a) Bernard S, Enayati A, Redwood L, Roger H, Binstock T. Medical Hypotheses. 2001;56:462–471. doi: 10.1054/mehy.2000.1281. [DOI] [PubMed] [Google Scholar]; (b) Baker JP. Am J Public Health. 2008;98:244–253. doi: 10.2105/AJPH.2007.113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]; (b) Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. The Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 9.(a) Sommar J, Gardfeldt K, Strömberg D, Feng X. Atmos Environ. 2001;35:3049–3054. [Google Scholar]; (b) Hall B. Water, Air, Soil Pollut. 1995;80:301–315. [Google Scholar]
- 10.Hamdy MK, Noyes OR. Appl Microbiol. 1975;30:424–432. doi: 10.1128/am.30.3.424-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomiyasu T, Okada M, Imura R, Sakamoto H. Sci Total Environ. 2003;304:221–230. doi: 10.1016/S0048-9697(02)00571-5. [DOI] [PubMed] [Google Scholar]
- 12.Erickson BE. Chem Eng News. 2008;86:47–49. [Google Scholar]
- 13.United States Environmental Protection Agency. Clean air mercury rule, 40 CFR Parts 60, 63, 72, and 75. 2005. [Google Scholar]
- 14.Solomon GM, Janssen S. JAMA. 2010;304:1118–1119. doi: 10.1001/jama.2010.1254. [DOI] [PubMed] [Google Scholar]
- 15.(a) Ghaedi M, Fathi MR, Shokrollahi A, Shajarat F. Anal Lett. 2006;39:1171–1185. [Google Scholar]; (b) Taylor A, Branch S, Halls D, Patriarca M, White M. J Anal At Spectrom. 2002;17:414–455. [Google Scholar]
- 16.Beauchemin D. Anal Chem. 2008;80:4455–4486. doi: 10.1021/ac8006945. [DOI] [PubMed] [Google Scholar]
- 17.Hutchison AR, Atwood DA. J Chem Crystallogr. 2003;33:631–645. [Google Scholar]
- 18.Thakur JS, Prinja S, Singh D, Rajwanshi A, Prasad R, Parwana HK, Kumar R. J Epidemiol Commun Health. 2010;64:148–154. doi: 10.1136/jech.2008.078568. [DOI] [PubMed] [Google Scholar]
- 19.(a) Lee MH, Lee SW, Kim SH, Kang C, Kim JS. Org Lett. 2009;11:2101–2104. doi: 10.1021/ol900542y. [DOI] [PubMed] [Google Scholar]; (b) Nolan EM, Lippard SJ. J Am Chem Soc. 2007;129:5910–5918. doi: 10.1021/ja068879r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yoon S, Albers AE, Wong AP, Chang CJ. J Am Chem Soc. 2005;127:16030–16031. doi: 10.1021/ja0557987. [DOI] [PubMed] [Google Scholar]; (d) Yoon S, Miller EW, He Q, Do PH, Chang CJ. Angew Chem, Int Ed. 2007;46:6658–6661. doi: 10.1002/anie.200701785. [DOI] [PubMed] [Google Scholar]
- 20.(a) Nolan EM, Lippard SJ. Chem Rev. 2008;108:3443–3480. doi: 10.1021/cr068000q. [DOI] [PubMed] [Google Scholar]; (b) Kumar A, Pandey PS. Tetrahedron Lett. 2009;50:5842–5845. [Google Scholar]; (c) Lee DN, Kim GJ, Kim HJ. Tetrahedron Lett. 2009;50:4766–4768. [Google Scholar]; (d) Loe-Mie F, Marchand G, Berthier J, Sarrut N, Pucheault M, Blanchard-Desce M, Vinet F, Vaultier M. Angew Chem, Int Ed. 2010;49:422–425. doi: 10.1002/anie.200905037. [DOI] [PubMed] [Google Scholar]; (e) Du JJ, Fan JL, Peng XJ, Sun PP, Wang JY, Li HL, Sun SG. Org Lett. 2010;12:476–479. doi: 10.1021/ol902590g. [DOI] [PubMed] [Google Scholar]; (f) Fuertes P, Moreno D, Cuevas JV, García-Valverde M, Torroba T. Chem–Asian J. 2010;5:1692–1699. doi: 10.1002/asia.201000063. [DOI] [PubMed] [Google Scholar]; (g) Cho YS, Ahn KH. Tetrahedron Lett. 2010;51:3852–3854. [Google Scholar]; (h) Hu J, Zhang M, Yu LB, Ju Y. Bioorg Med Chem Lett. 2010;20:4342–4345. doi: 10.1016/j.bmcl.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 21.(a) Zhang JF, Kim JS. Anal Sci. 2009;25:1271–1281. doi: 10.2116/analsci.25.1271. [DOI] [PubMed] [Google Scholar]; (b) Fan JL, Guo KX, Peng XJ, Du JJ, Wang JY, Sun SG, Li HL. Sensors Actuat B-Chem. 2009;142:191–196. [Google Scholar]; (c) Kim JH, Kim HJ, Kim SH, Lee JH, Do JH, Kim HJ, Lee JH, Kim JS. Tetrahedron Lett. 2009;50:5958–5961. [Google Scholar]; (d) Atilgan S, Kutuk I, Ozdemir T. Tetrahedron Lett. 2010;51:892–894. [Google Scholar]; (e) Song CX, Zhang XL, Jia CY, Zhou P, Quan X, Duan CY. Talanta. 2010;81:643–649. doi: 10.1016/j.talanta.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 22.Li CY, Zhang XB, Qiao L, Zhao Y, He CM, Huan SY, Lu LM, Jian LX, Shen GL, Yu RQ. Anal Chem. 2009;81:9993–10001. doi: 10.1021/ac9018445. [DOI] [PubMed] [Google Scholar]
- 23.United States Environmental Protection Agency. Method 1631, Revision E: Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. 2002. [Google Scholar]
- 24.Morton J, Carolan VA, Gardiner PHE. J Anal At Spectrom. 2002;17:377–381. [Google Scholar]
- 25.Harris HH, Pickering IJ, George GN. Science. 2003;301:1203–1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- 26.Song F, Watanabe S, Floreancig PE, Koide K. J Am Chem Soc. 2008;130:16460–16461. doi: 10.1021/ja805678r. [DOI] [PubMed] [Google Scholar]
- 27.United States Environmental Protection Agency. EPA 816-F-09-004. 2009. [Google Scholar]
- 28.Gigg J, Gigg R. J Chem Soc C. 1966:82–86. doi: 10.1039/j39660001872. [DOI] [PubMed] [Google Scholar]
- 29.Gigg R, Warren CD. J Chem Soc C. 1968:1903–1911. [Google Scholar]
- 30.Song F, Garner AL, Koide K. J Am Chem Soc. 2007;129:12354–12355. doi: 10.1021/ja073910q. [DOI] [PubMed] [Google Scholar]
- 31.(a) Garner AL, Koide K. Chem Commun. 2009:83–85. doi: 10.1039/b817220j. [DOI] [PubMed] [Google Scholar]; (b) Garner AL, Koide K. Chem Commun. 2009:86–88. doi: 10.1039/b814197e. [DOI] [PubMed] [Google Scholar]; (c) Garner AL, Song F, Koide K. J Am Chem Soc. 2009;130:5163–5171. doi: 10.1021/ja808385a. [DOI] [PubMed] [Google Scholar]; (d) Garner AL, St Croix CM, Pitt BR, Leikauf GD, Ando S, Koide K. Nat Chem. 2009;1:316–321. doi: 10.1038/nchem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Song F, Carder EJ, Kohler CC, Koide K. Chem–Eur J. 2010 doi: 10.1002/chem.201001316. published online. [DOI] [PubMed] [Google Scholar]
- 32.Sparano BA, Shahi SP, Koide K. Org Lett. 2004;6:1947–1949. doi: 10.1021/ol049537y. [DOI] [PubMed] [Google Scholar]
- 33.Koide K, Song F, de Groh ED, Garner AL, Mitchell VD, Davidson LA, Hukriede NA. ChemBioChem. 2008;9:214–218. doi: 10.1002/cbic.200700565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prosser TJ. J Am Chem Soc. 1961;83:1701–1704. [Google Scholar]
- 35.Morse H. Z Physik Ch. 1902;41:709–734. [Google Scholar]
- 36.Taken from the Allegheny River, Pittsburgh, Pennsylvania. The mercury content was determined by the cold vapor atomic absorption (Department of Environmental Protection, Bureau of Laboratories, Harrisburg, Pennsylvania, USA). We thank Dr. Taru Upadhyay for this analysis.
- 37.Allen TL. J Chem Phys. 1957;26:1644–1647. [Google Scholar]
- 38.Forbes GS, Cole HI. J Am Chem Soc. 1921;43:2492–2497. [Google Scholar]
- 39.Zhang FS, Nriagu JO, Itoh H. Water Res. 2005;39:389–395. doi: 10.1016/j.watres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Ritter JA, Bibler JP. Water Sci Technol. 1992;25:165–172. [Google Scholar]
- 41.Actual mercury-contaminated discharged water samples could not be obtained from an environmental agency presumably due to the safety for shipping.
- 42.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 43.Although the signals of samples with Hg(II) were lower than expected, it should be noted that organic compounds spiked with Hg(II) did not affect the reaction. A higher concentration of DMSO (1.3% in the reaction solutions; 26 times the concentration of the general method that we used in this study) seems to be the reason of these lower signals.
- 44.MacDougall D, et al. Anal Chem. 1980;52:2242–2249. [Google Scholar]
- 45.Santra M, Ryu D, Chatterjee A, Ko SK, Shin I, Ahn KH. Chem Commun. 2009:2115–2117. doi: 10.1039/b900380k. [DOI] [PubMed] [Google Scholar]
- 46.Lavis LD, Rutkoski TJ, Raines RT. Anal Chem. 2007;79:6775–6782. doi: 10.1021/ac070907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arata M, Miura T, Chiba K. Org Lett. 2007;9:4347–4350. doi: 10.1021/ol7019845. [DOI] [PubMed] [Google Scholar]
- 48.Ei-Shishtawy RM, Almeida P. Tetrahedron. 2006;62:7793–7798. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.