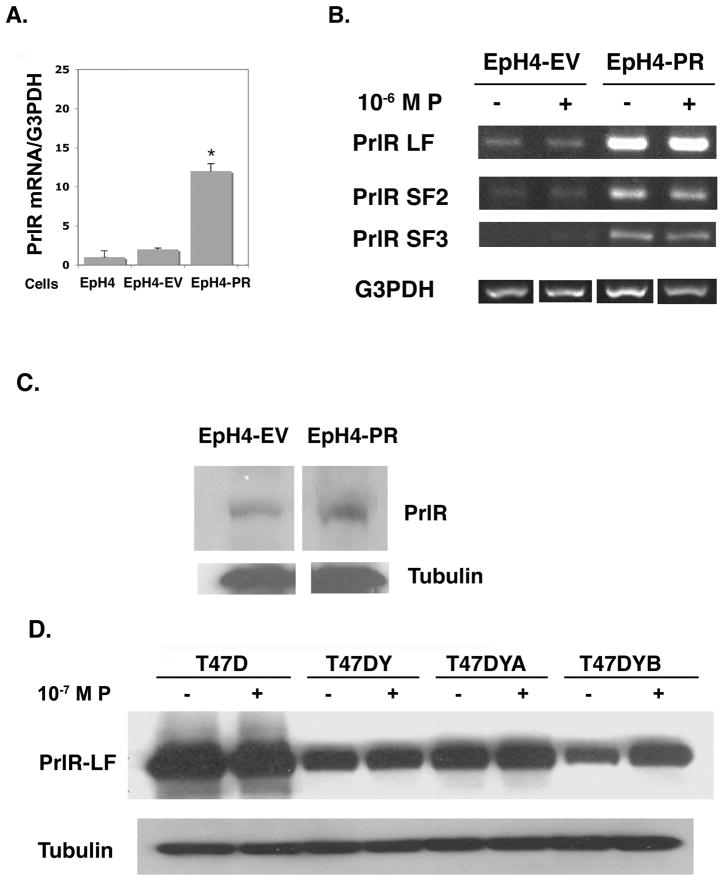
Fig. 1.
Increased expression of PrlR mRNA and protein in EpH4-PR cells and T47D cells treated with progesterone.
A. Parental EpH4, EpH4-EV and EpH4-PR cells were plated and maintained in DMEM growth media containing 10% FBS for 48 hr. Media was then changed to 5% CSS and, 24 hr. later, cells were collected. RNA was extracted, DNase-treated, and subjected to RT-PCR using PCR primers specific for mouse PrlR extracellular domain and G3PDH. PCR products were resolved by gel electrophoresis and quantified using NIH Image J. Densitometric histograms from three separate determinations are expressed relative to G3PDH. * = p 0.001 relative to EpH4-EV.
B. EpH4-EV and EpH4-PR cells were plated and maintained in growth media containing 10% FBS for 48 hr. Media were then changed to 5% CSS ± 10−6M P and 24 hr. later cells were collected. RNA was extracted, DNase-treated, and subjected to RT-PCR using PCR primers specific for mouse PrlR LF, SF1, SF2, SF3, and G3PDH. PCR products were resolved by gel electrophoresis. No message was observed for SF1. Data shown are representative of 3 separate experiments.
C. EpH4-EV and EpH4-PR cells were plated, grown, and treated as in (A) above. Cells were then collected in homogenization buffer and whole cell lysates were subjected to western blot analysis with M2.5 antibody that detects the extracellular domain of the mouse PrlR. Nitrocellulose blots were stripped and probed with anti-tubulin as a loading control.
D. T47D, T47DY, T47DYA and T47DYB cells were plated in RPMI growth media containing 5% FBS. The next day, cells were switched to media containing 1% CSS for 24hr and then to media containing 0.1% CSS ± 10−7 M P for up to 48 hr. Whole cell lysates were subject to western blot analysis with antibody detecting the extracellular domain of the PrlR. Data shown are after 24 hr treatment with P. Blots were stripped and probed with anti-tubulin as a loading control.