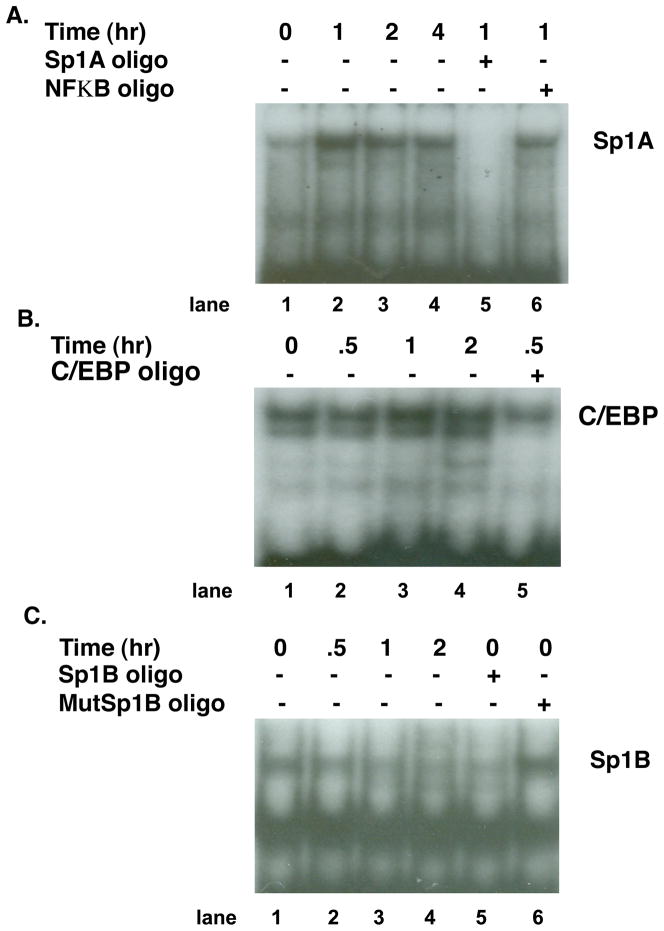
Fig. 4.
Effect of P on recruitment of nuclear proteins from T47D cells to transcription factor response sites on the PrlR promoter.
A. T47D cells were plated in growth media containing 5% FBS. Over a 4 day period, serum concentration was stepped down, first to 1% and then to 0.1% CSS. On day 5, cells were treated with 10−8 M P or vehicle for 0, 1, 2 or 4 hr. Nuclear proteins were incubated with a 32P-labeled oligonucleotide probe with sequence identical to the Sp1A response site prior to electrophoresis. A faint protein complex bound to probe in the absence of P (0 hr; lane 1) showed induction with P treatment with time (lanes 2–4). Specificity of binding is demonstrated by competition with excess unlabeled Sp1A oligo (lane 5) and lack of competition with the unlabeled unrelated NFκB oligo (lane 6).
B. T47D cells were plated and cultured as in (A). On day 5, cells in media containing 0.1% CSS were treated with 10−8 M P or vehicle for 0, 0.5, 1, or 2 hr. Nuclear proteins were incubated with a 32P-labeled oligonucleotide probe with sequence identical to the C/EBP response site prior to electrophoresis. A protein complex bound to probe in the absence of P (0 hr; lane 1) showed induction with P treatment with time (lanes 2–4). Specificity of binding is demonstrated by competition with excess unlabeled C/EBP oligo (lane 5).
C. Nuclear extracts obtained as in (B) were incubated with 32P-labeled oligonucleotide probe with sequence identical to the Sp1B response site. Specific complexes for Sp1B do not appear to be upregulated by P treatment. Specificity of binding is demonstrated by competition with an excess of unlabeled Sp1B oligo (lane 5) and lack of competition with the unlabeled mutated Sp1B oligo (lane 6).