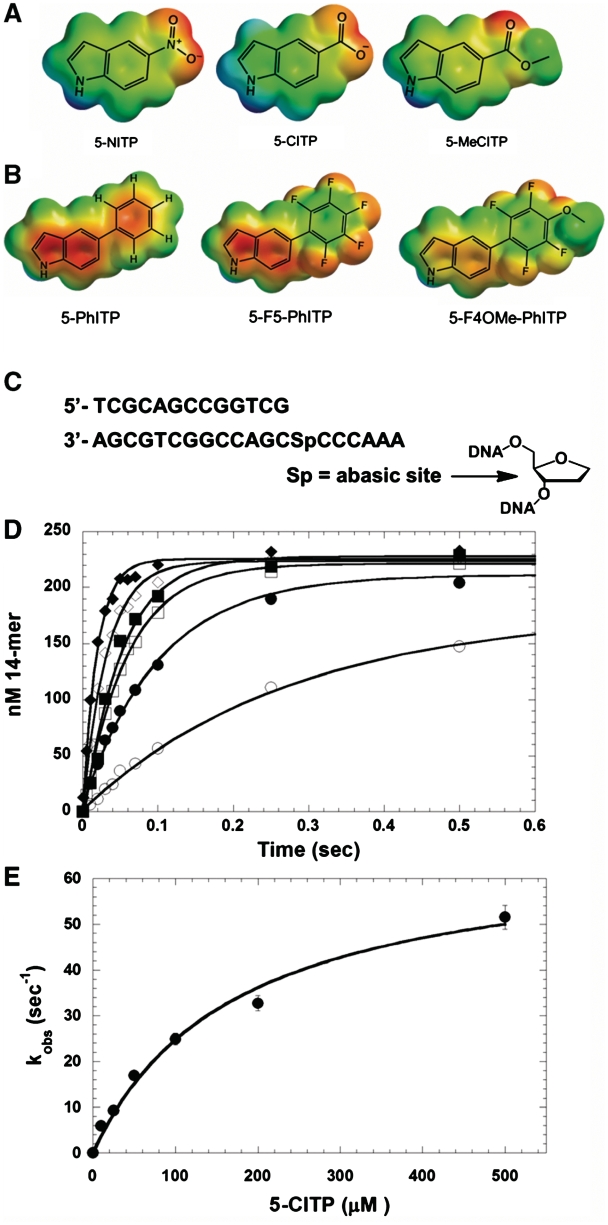
Figure 2.
Structures of (A) 5-NITP, 5-CITP and 5-MeCITP; (B) 5-PhITP, 5-F5-PhITP and 5-F4OMe-PhITP. ‘For clarity, only the nucleobases of the isosteric 5-substituted indolyl nucleotide analogues are shown’. (C) Nucleotide sequence of the DNA substrate used in kinetic studies. (D) Dependency of the apparent burst rate constant on the concentration of 5-CITP as measured under single-turnover conditions. Assays were performed using 500 nM gp43 exo−, 250 nM 13/20SP-mer, 10 mM Mg(OAc)2 and 5-CITP in variable concentrations of 10 (open circle), 25 (closed circle), 50 (open square), 100 (closed square), 200 (open diamond) and 500 µM (closed diamond). (E) Observed rate constants for incorporation (closed circle) were plotted against 5-CITP concentration. A fit of the data to the Michaelis–Menten equation yields a kpol of 67 ± 5 s−1 and a Kd, app of 172 ± 31 µM.