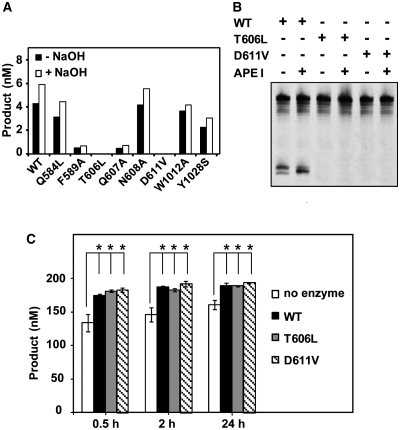
Figure 3.
T606 and D611 are essential for ROS1 DNA glycosylase activity. (A) The generation of incision products was measured by incubating purified WT ROS1 or mutant variants (20 nM) at 30°C for 2 h with a double-stranded oligonucleotide substrate (20 nM) containing a single 5-meC:G pair. Samples were treated with or without NaOH 100 mM, and immediately transferred to 90°C for 10 min. Products were separated in a 12% denaturing polyacrylamide gel and the amounts of incised oligonucleotide were quantified by fluorescent scanning. (B) Purified WT ROS1 or mutant variants (20 nM) were incubated at 30°C for 2 h with a double-stranded oligonucleotide substrate (20 nM) containing a single 5-meC:G pair, either in the absence or the presence of human APE I (5 U), as indicated. Products were separated in a 12% denaturing polyacrylamide gel and the incised products were detected by fluorescent scanning. (C) A double-stranded oligonucleotide substrate containing an AP site opposite G (200 nM) was incubated at 30°C either in the absence of enzyme or in the presence of purified WT ROS1, T606L or D611V (100 nM). Reactions were stopped at the indicated times, products were separated in a 12% denaturing polyacrylamide gel and the amount of incised oligonucleotide was quantified by fluorescent scanning. Values are means ± SE (error bars) from two independent experiments. The asterisks indicate that the incision levels were significantly different (P < 0.05) from those observed in the absence of enzyme. The respective P-values were calculated using a Student’s unpaired t-test.