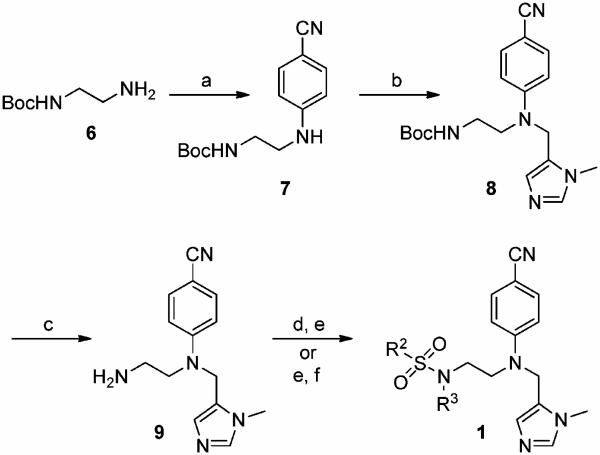
Scheme 1a.
a (a) para-Fluorobenzonitrile, DIPEA, DMSO, 120 °C, 48 h, 89%; (b) (1) LDA, THF, −78 °C, 30 min, (2) 5-chloromethyl-1-methyl-1H-imidazole · HCl,29 NaH, −78 °C, 1 h, 52% (98% brsm); (c) TFA-CH2-Cl2, 1:1, rt, 30min, 99%; (d) (1) R2CHO, AcOH, 4 Å MS, MeOH, rt, 30min, (2) NaCNBH3, rt, 16 h, 72–84%; (e) R2SO2Cl,DIPEA,CH3CN, 0 °C → rt, 16 h, 82–93%; ((f) R3Br, Cs2CO3, DMF, rt, 16 h, 79–82%.