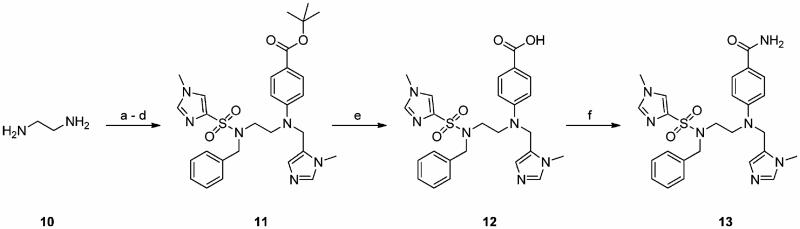
Scheme 2a.
a (a) tert-Butyl para-fluorobenzoate, DMSO, 120 °C, 24 h, 96%; (b) 1-methyl-1H-imidazole-4-sulfonyl chloride, DIPEA, CH3CN, 0 °C → rt, 12 h, 90%; (c) BnBr, Cs2CO3, DMF, rt, 16 h, 92%; (d) (1) NaH, DMF, 0 °C, 30 min, (2) 5-chloromethyl-1-methyl-1H-imidazole · HCl,29b 0 °C → rt, 3 h, 76%; (e) TFA-CH2Cl2, 1:1, 3 h, rt, 96%; (f) NH4Cl, HBTU, DIPEA, DMF, rt, 16 h, 89%.