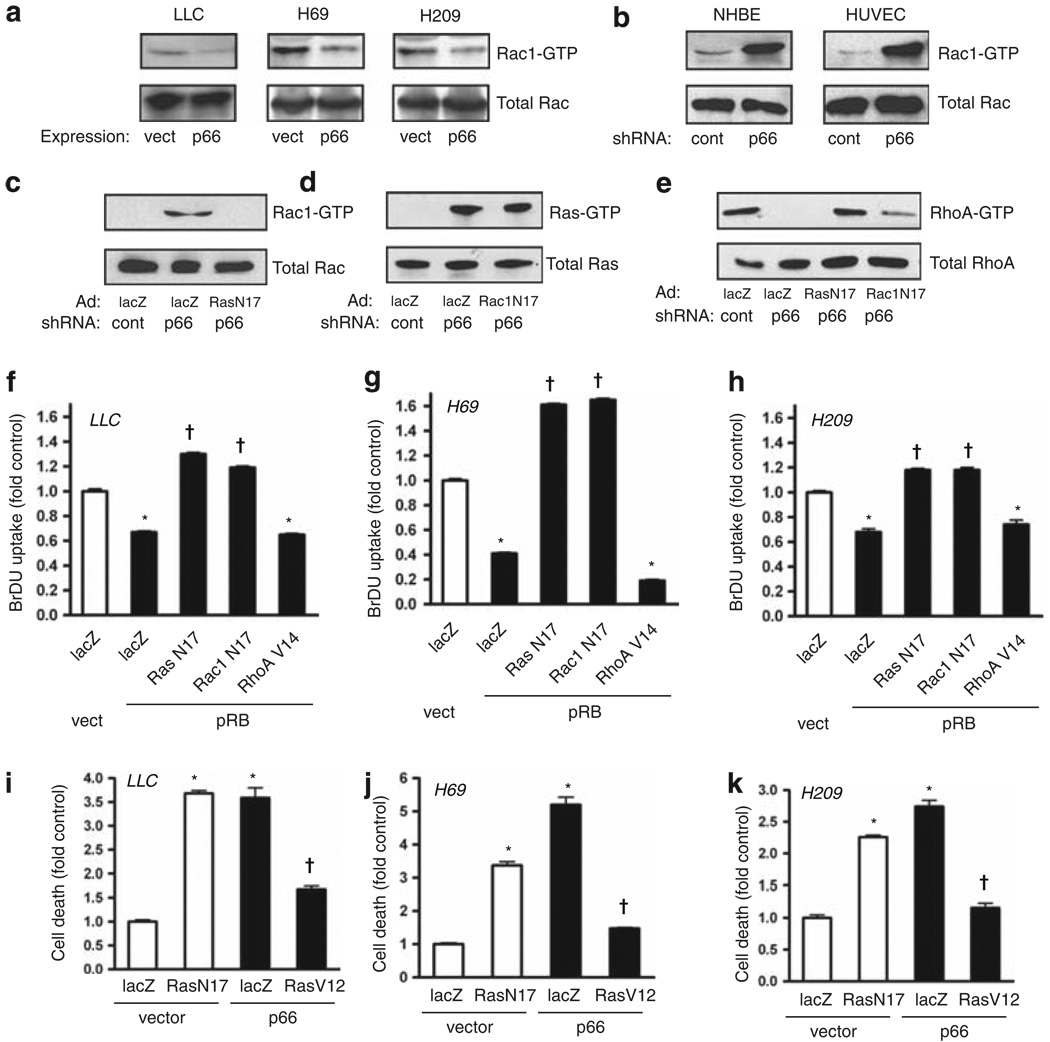
Figure 5.
Loss of p66Shc suppresses RhoA through Ras and Rac1 activation. (a) Re-expression of p66Shc through lentiviral delivery suppressed active Rac1, assessed by pulldown. (b) Knockdown of p66Shc by shRNA caused intense activation of Rac1 in NHBE cells and human umbilical vein endothelial cells (HUVECs). (c–e) Ras(N17) or Rac1(N17) were expressed and p66Shc knocked down as indicated in NHBE cells, and active Rac1, Ras and RhoA were assessed by pulldown. (f–h) Dominant negatives Ras(N17) and Rac1(N17) and constitutively active RhoA(V14) were delivered by adenovirus; pRB was expressed by lentiviral delivery in LLC, H69 and H209 cells. pRB suppressed BrDU uptake (*P < 0.001 from lacZ/vector control), whereas both Ras(N17) and Rac1(N17) restored proliferation above baseline (†P < 0.001 from lacZ/pRB). Active RhoA(V14) did not rescue proliferation from pRB. Mean ± s.e.m. of six determinations is shown. (i–k) LLC, H69 and H209 cells were grown in low attachment plates following transduction as indicated. DNA fragmentation was assessed 16 h later. Suppression of endogenous Ras with Ras(N17) caused death in floating cells for all three cell lines to levels comparable to those caused by p66Shc expression (*P < 0.001 from lacZ/vector control). In contrast, active Ras(V12) rescued all three cells from p66Shc-induced anoikis (†P < 0.001 from respective lacZ/p66 groups). Mean ± s.e.m. of four determinations is shown.