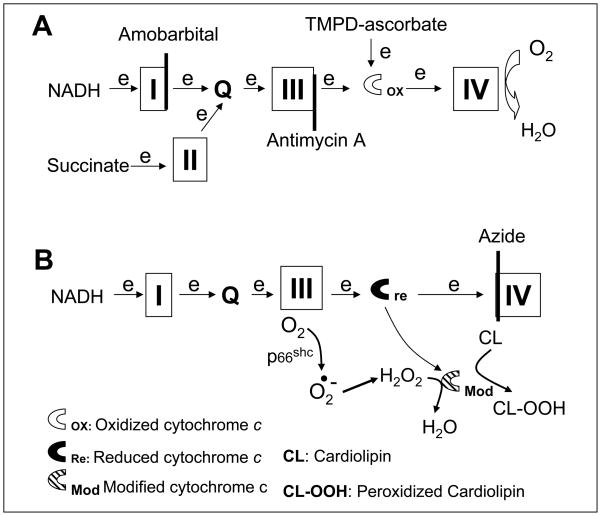
Figure 2. Depiction of the potential sites in the electron transport chain that lead to mitochondrial damage during ischemia.
Electron flux from NADH enters the electron transport chain (ETC) at complex I and proceeds complex I→III→cytochrome c→ IV. Succinate is oxidized by complex II and results in electron flow from complex II →III→ cytochorme c→cytochrome oxidase. Amobarbital blocks electron transport distal within complex I (Panel A) and protect against ischemia-mediated mitochondrial damage. Thus, the key site of ETC-driven injury must lie distal to complex I. Previous work shows that antimycin A inhibits at cytochrome b of complex III (Panel A) and it prevents cardiolipin depletion and the loss of cytochrome c from cardiac mitochondria during ischemia [9, 10]. This further indicates the ischemia-damaged site is distal to complex III (Panel A). Ischemia damages complex III and increases the production of reactive oxygen species [5, 34, 35]. The increased superoxide is converted to H2O2. H2O2 is reduced to H2O by cytochrome c-cardiolipin peroxidase (Modified cytochrome c), and simultaneously cardiolipin is oxidized to peroxidized cardiolipin (CL-OOH) that favors cytochrome c detachment from the inner mitochondrial membrane and release from mitochondria as observed in the present study (Panel B). Azide inhibits complex IV and maintains cytochrome c in the reduced state that favors H2O2 generation via the actions of p66shc(Panel B).