Figure 1.
Major Cellular Interactions and Regulatory Pathways Involved in IgA Responses to Intestinal Antigens
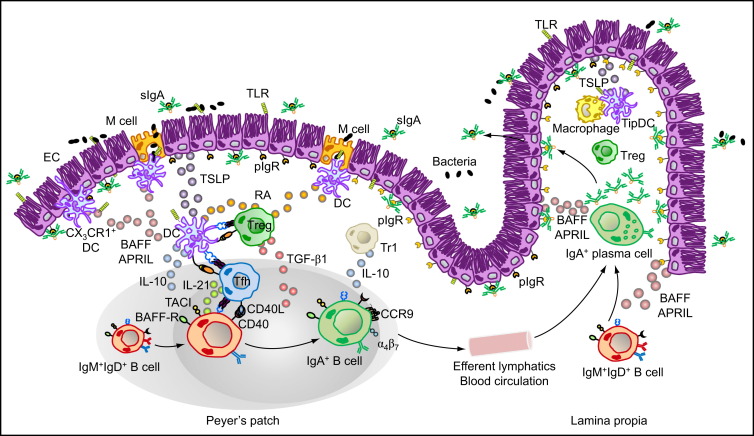
M cells from PPs sample commensal and vaccine antigens from the intestinal lumen and deliver them to subepithelial DCs. Antigen sampling is also carried out by CX3CR1+ DCs that project dendrites into the intestinal lumen across ECs. These cells release immunoregulatory (TSLP) and IgA-inducing (BAFF and APRIL) molecules upon sensing microbial signatures through TLRs and NLRs. TSLP stimulates the formation of tolerogenic DCs that suppress proinflammatory Th1 responses and induce noninflammatory Treg and Th2 responses by releasing IL-6, IL-10, TGF-β1, and RA. Treg cells may further differentiate into TFH cells, which together with Treg and perhaps Th2 cells stimulate IgA CSR and production by stimulating naive IgM+IgD+ B cells through CD40L, TGF-β1, IL-6, IL-10, and IL-21. In the presence of RA, IgA-expressing B cells emerging from mucosal germinal centers acquire expression of gut-homing receptors such as CCR9 and α4β7, which direct subsequent B cell migration to the intestinal LP through efferent lymphatics, regional mesenteric lymph nodes, and blood circulation. In the LP, IgA-expressing B cells differentiate into IgA-secreting plasma cells that secrete IgA dimers. Interaction of IgA dimers with the pIgR results into IgA transcytosis and formation of a secretory IgA (SIgA) complex that binds antigen in the intestinal lumen. The LP also contains IL-10-producing macrophages and BAFF-APRIL-nitric oxide-producing TipDCs whose development is promoted by microbial and epithelial factors such as TSLP. TipDCs and macrophages would enhance local IgA production by triggering CSR and stimulating plasma cell survival.