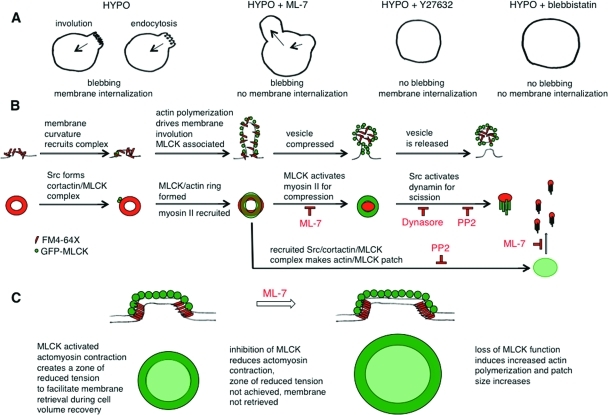
FIGURE 11:
Models of MLCK and Src in control of membrane dynamics during osmotic stress. (A) In response to hypotonic swelling, a bleb membrane may be retracted with retrieval of added surface area by actomyosin-mediated involution or endocytosis. When MLCK is inhibited with ML-7, a bleb on a swollen cell can appear to collapse, but this occurs concomitantly with formation of another bleb and the total membrane area is not decreased. The inward membrane movement under these conditions is likely due to ROCK-mediated myosin II–dependent tension because inhibition of ROCK (with Y27632) and of myosin II (with blebbistatin) leads to cell swelling without bleb formation. Under hypotonic conditions when MLCK tension is reduced, the imbalance of unopposed ROCK tension pulls against cortical actin/membrane linkages to lead to bleb formation. (B) MLCK and Src could regulate volume-sensitive membrane retrieval at several levels, in analogy to the roles ascribed to actin and myosins in endocytic vesicle formation and internalization (Liu et al., 2010). The first row of schematics indicates a potential set of intermediate stages of membrane retrieval, and the second row the correlated structures from our imaging data. Sites of specialized membrane curvature, which form in response to swelling, may recruit a Src/MLCK/cortactin complex that could promote cortactin-regulated actin polymerization to involute the membrane and stabilize it with an actin coat. Concomitant MLCK and myosin II recruitment would allow MLCK-activated myosin II to create zones of reduced membrane tension to allow endocytic machinery to act to facilitate cell volume recovery. In the macroscopic mode of membrane retrieval (rings), MLCK-activated myosin II could then drive actin coat compression (Yu and Bement, 2007). MLCK-activated myosin II may potentially also facilitate vesicle neck constriction (Bhat and Thorn, 2009) and act together with Src-activated dynamin, which is thought to be necessary but not sufficient for fission (Liu et al., 2010). Inhibition of MLCK might be expected to slow the coat compression step of the macroscale endocytic (ring) mode and may influence a shift to the microscale (patch). Src function is required for the activation of dynamin, and thus inhibition of Src and of dynamin would be expected to lead to accumulation of prescission intermediates. (C) In contrast to rings, patches require the kinase function of Src for their formation. Once formed, patches require MLCK kinase activity to maintain their shape and size. We propose that the concomitant recruitment of actin and MLCK to patches represents the formation of an actomyosin network that stabilizes the patch periphery against the increased membrane tension of a swollen cell. In the absence of MLCK kinase function with sustained elevated membrane tension, patches enlarge. Thus Src-activated MLCK structures may provide fine-tuning of membrane tension to permit membrane retrieval in the face of hydrostatic pressure.