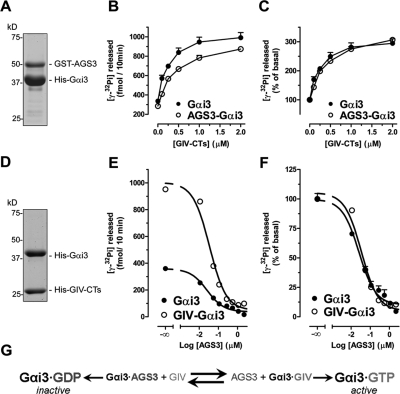
FIGURE 2:
Gαi3 activity is reversibly modulated by GIV (a GEF) and AGS3 (a GDI). (A) Preparation of Gαi3–AGS3 complexes. His-Gαi3:GST-AGS3 (465–650) complexes were purified (4:1 stoichiometry) by glutathione-agarose affinity chromatography. An aliquot of the purified complex was separated by SDS–PAGE and stained with Coomassie blue. (B, C) GIV efficiently promotes G protein activation in the Gαi3–AGS3 complex. The steady-state GTPase activity of purified His-Gαi3 (50 nM) alone (closed circles) or in complex with GST-AGS3 (open circles) was determined in the presence of the indicated amounts (0, 0.1, 0.25, 0.5, 1, and 2 μM) of purified His-GIV-CTs (aa 1660–1870, containing the GEF motif responsible for Gαi binding) by quantification of the amount of [γ-32P]GTP (0.5 μM, ∼50 cpm/fmol) hydrolyzed in 10 min. Data are expressed as fmol of radioactive phosphate (32Pi) released (absolute GTPase activity, B). The same data were normalized to the GTPase activity in the absence of His-GIV-CTs and expressed as % of 32Pi released (fold activation, C) by the G protein alone (closed circles) or in complex with GST-AGS3 (open circles) in the absence of His-GIV-CTs. Results are shown as mean ± SD of a representative experiment out of three performed in duplicate. (D) Preparation of Gαi3:GIV-CT complexes. His-Gαi3:His-GIV-CTs (aa 1660–1870) complexes were purified (1:1 stoichiometry) by gel filtration chromatography. An aliquot of the purified complex was separated by SDS–PAGE and stained with Coomassie blue. (E, F) AGS3 efficiently blocks G protein activation in the Gαi3–GIV complex. The steady-state GTPase activity of purified His-Gαi3 (50 nM) alone (closed circles) or in complex with His-GIV-CTs (open circles) was determined in the presence of the indicated amounts (0, 0.11, 0.055, 0.11, 0.275, 0.55, 1.1, and 2.2 μM) of purified His-AGS3 (aa 424–650) by quantification of the amount of [γ-32P]GTP (0.5 μM, ∼50 cpm/fmol) hydrolyzed in 10 min. Data are expressed as fmol radioactive phosphate (32Pi) released (absolute GTPase activity, E). The same data were normalized to the GTPase activity in the absence of His-AGS3 and expressed as % of 32Pi released (fold activation, F) by the G protein alone (closed circles) or in complex with His-GIV-CTs (open circles) in the absence of His-AGS3. Results are shown as mean ± SD of a representative experiment out of three performed in duplicate. (G) Schematic representation of the reversible regulation of Gαi3 activity by AGS3 GDI and GIV GEF.