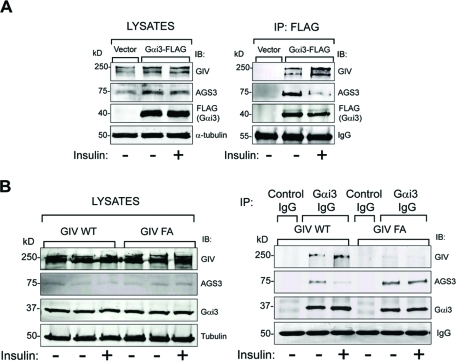
FIGURE 4:
GIV’s GEF motif is required for insulin to trigger a shift in Gαi3 binding from AGS3 to GIV. (A) Gαi3 coimmunoprecipitates with AGS3 in serum-starved cells and with GIV when cells are insulin stimulated. Cos7 cells transiently transfected with FLAG-tagged Gαi3 (Gαi3-FLAG) or vector control were serum starved (-) and stimulated with 100 nM insulin (+) for 15 min before lysis. Equal aliquots of cell lysates (left panel) were incubated with anti-FLAG mAb. Immunoprecipitated complexes (right panel) were analyzed for GIV, AGS3, and FLAG (Gαi3) by immunoblotting (IB). In serum-starved cells (−), Gαi3-bound immune complexes are enriched in AGS3, whereas after insulin treatment (+) these immune complexes are depleted of AGS3 and enriched in GIV. Identical observations were made after EGF treatment (Supplemental Figure S4). (B) Changes in the abundance of Gαi–AGS3 complexes in response to insulin require an intact GEF motif in GIV. Immunoprecipitation was carried out on lysates of serum-starved and insulin-stimulated control, GIV-WT, and GIV-FA cells with anti-Gαi3 IgG, and the immune complexes were analyzed for Gαi3, AGS3, and GIV by IB. In GIV-WT cells, the amount of Gαi3-bound GIV increased and the amount of Gαi3-bound AGS3 decreased upon insulin treatment. In GIV-FA cells (GEF-deficient mutant), GIV does not coimmunoprecipitate with Gαi3, and the extent of Gαi3-bound AGS3 remains unaltered after insulin treatment.