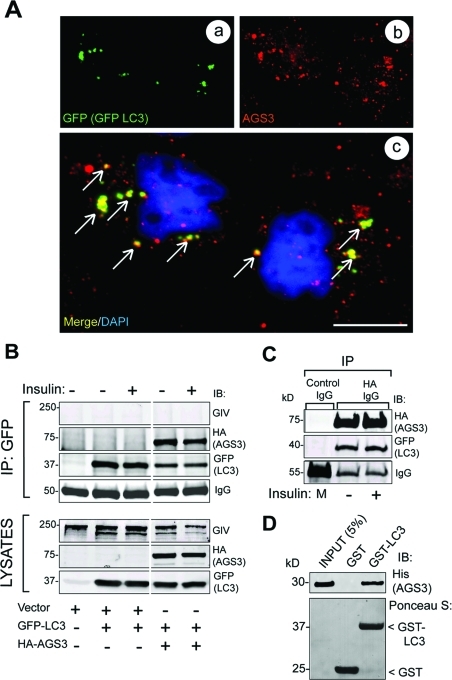
FIGURE 5:
AGS3 localizes to autophagosomes and interacts with the autophagy effector LC3. (A) GFP-LC3 and endogenous AGS3 partially colocalize on vesicular structures in serum-starved cells. HeLa cells expressing GFP-LC3 were serum starved (0.2% FBS) in the presence of leupeptin and pepstatin. Cells were permeabilized with 0.05% saponin, fixed, stained for LC3 (GFP, green, a) and for AGS3 (red, b), and analyzed by confocal microscopy. The merged panel (c) shows that AGS3 colocalizes with GFP-LC3 (yellow pixels, arrows) on some of the punctate autophagic structures. Bar = 10 μM. (B) AGS3 coimmuno-precipitates with GFP-LC3 in vivo. Cos7 cells transiently cotransfected with GFP-LC3, and either AGS3-HA or vector control were serum starved as in (A) and then treated with insulin before lysis. Equal aliquots of cell lysates (bottom panels) were treated with GFP mAb, and immuno-precipitated complexes (top panels) were analyzed for AGS3 (HA), LC3 (GFP), and GIV by immunoblotting (IB). AGS3 and LC3 interact both in serum-starved and insulin-stimulated cells. (C) LC3 coimmunoprecipitates with AGS3-HA. Lysates of Cos7 cells coexpressing GFP-LC3 and AGS3-HA (as above) were treated with HA mAb, and a 1:1 mix (M) of the two lysates was treated with mouse preimmune IgG. Immunoprecipitated complexes were analyzed for AGS3 (HA) and LC3 (GFP) by IB. AGS3 and LC3 coimmunoprecipitate in both serum-starved and insulin-stimulated cells. (D) His-AGS3 (aa 424–650) directly interacts with GST-LC3. Equal aliquots (15 μg) of GST or GST-LC3 were used in pull-down assays with 3 μg His-AGS3. Bound proteins were visualized by IB for His-AGS3. AGS3 binds GST-LC3 but not the GST control.