FIGURE 6:
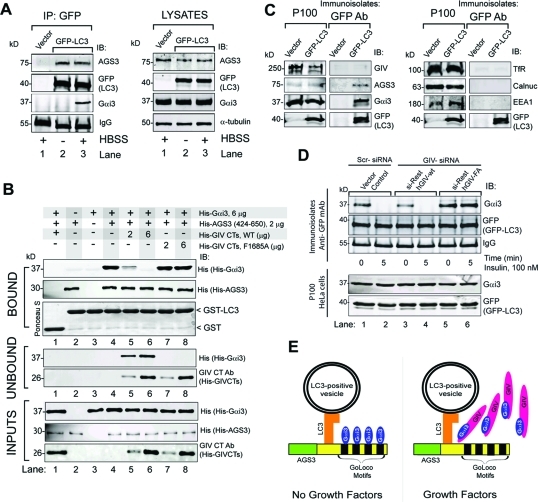
AGS3 links Gαi3 to LC3 by forming a ternary (Gαi3–AGS3–LC3) complex, and GIV’s GEF motif is required for the release of Gαi3 from this complex. (A) Endogenous AGS3 and Gαi3 coimmunoprecipitate with GFP-LC3. HeLa cells expressing GFP-LC3 or vector control were maintained in either 10% FBS (−) or Hank’s balanced salt solution (HBSS) media (+) in the presence of leupeptin and pepstatin for 12 h before lysis. Immunoprecipitation was carried out on equal aliquots of cell lysates (right panel) using anti-GFP IgG, and the bound immune complexes (lanes 1–3) were analyzed for LC3 (GFP-LC3), Gαi3, and AGS3 by immunoblotting (IB, left panel). AGS3 is present in LC3-bound complexes under both 10% serum (−) and HBSS-starved (+) conditions (lanes 2 and 3), whereas Gαi3 is present exclusively upon starvation (lane 3). Neither AGS3 nor Gαi3 were detected in GFP immunoprecipitates from cells transfected with vector control (lane 1). (B) His-Gαi3 binds GST-LC3 in the presence of His-AGS3 and can be displaced by His-GIV-CTs WT, but not by His-GIV-CTs FA. Equal aliquots of GST (lane 1) or GST-LC3 (15 μg, lanes 2–8) were incubated with either His-AGS3 alone (lane 2), His-Gαi3 alone (lane 3), or both proteins simultaneously (lanes 1 and 4–8) for 8–12 h at 4°C. After removing the unbound excess, GST–LC3-bound complexes were subjected to a second binding assay with increasing amounts (2 and 6 μg) of either WT His-GIV-CTs (lanes 5 and 6) or FA-mutant (lanes 7 and 8) (see bottom panel, “inputs”). GST–LC3-bound proteins were eluted with sample buffer and “bound” and “unbound” proteins were analyzed for Gαi3 (His-Gαi3), AGS3 (His-AGS3), and GIV (His-GIV-CTs). AGS3 directly binds LC3 (top panels, lane 2), but Gαi3 binds LC3 exclusively in the presence of AGS3 (top panels, compare lanes 3 and 4). His-GIV-CTs WT decreased the amount of His-Gαi3 bound to GST-LC3 (top panels, lanes 5 and 6) and concomitantly increased the amount of His-Gαi3 released into the supernatants (middle panel, lanes 5 and 6). In the case of mutant His-GIV-CTs FA, the amount of His-Gαi3 bound to GST-LC3 did not change (top panels, lanes 7 and 8). (C) Immunoisolation of GFP-LC3–positive vesicles. HeLa cells expressing GFP-LC3 or vector control were serum starved in the presence of leupeptin and pepstatin, homogenized, and processed for subcellular fractionation (see Materials and Methods). The crude membrane fraction (P100) was resuspended in homogenization buffer and incubated with anti-GFP IgG (mAb) and protein G beads. Equal aliquots of the P100 or immunoisolated LC3-positive membranes (immunoisolates: GFP antibody) were immunoblotted for GIV, AGS3, Gαi3, GFP-LC3 (left panels), and various organelle markers: transferrin receptor (Tfr, endosomes), EEA1 (early endosomes), calnuc (ER-Golgi), and GFP-LC3 (autophagosomes) (right panels). GFP-LC3, AGS3, and Gαi3 were detected in the GFP immunoisolates whereas GIV, Tfr, EEA1, and calnuc were not. Additionally, βCOP, cathepsin D, and lamin A, markers of ER-Golgi, lysosomes, and nuclear envelope, respectively, were also undetectable (unpublished data), indicating that LC3-positive membrane isolates were free of significant contaminants. (D) Upon insulin stimulation, Gαi3 is depleted from immunoisolated LC3-positive vesicles in GIV-WT but not GIV-FA cells. Control, GIV-WT, and GIV-FA HeLa cells were treated with scrambled (Scr) or GIV siRNA, followed by transient transfection with GFP-LC3 as in Figure 1B. Cells were serum starved and stimulated with insulin and homogenized, and equal aliquots of P100 fractions (100,000 × g pellets) (bottom panel) were incubated with GFP mAb for immunoisolation of GFP-LC3–positive membranes as in Figure 6C. Immunoisolates were analyzed for the presence of LC3 (GFP), Gαi3, AGS3, and GIV by IB. Gαi3 was detected in LC3-positive immunoisolates in all cell lines when serum starved (lanes 1, 3, and 5). Upon insulin treatment, Gαi3 was undetectable in controls (lane 2) and GIV-WT cells (lane 4) but present in GIV-FA cells (lane 6). (E) Schematic representation of the interplay between Gαi3, its modulators GIV and AGS3, and the autophagic effector LC3. In the absence of growth factors (left), AGS3 localizes to LC3-positive membranes by virtue of its direct, constitutive interaction within LC3. AGS3 recruits Gαi3 to these LC3-positive membranes on which Gαi3–AGS3–LC3 ternary complexes are assembled. On insulin stimulation (right), GIV binds Gαi3 and releases it from the Gαi3–AGS3–LC3 ternary complex assembled on membranes. This phenomenon is associated with inhibition of autophagosome formation and recovery from autophagy.