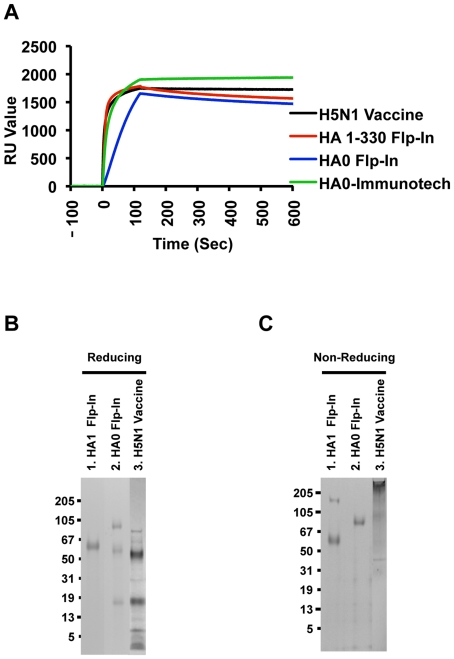
Figure 2. Characterization of purified HA proteins from 293 Flp-In cell.
(A) Proper protein folding as demonstrated by steady-state binding equilibrium analysis of conformational dependent human H5N1 neutralizing MAb FLA5.10 (10 µg/ml) to purified Flp-In expressed H5N1 HA1 proteins immobilized on a sensor chip through the free amine group, and onto a blank flow cell, free of peptide. Purified mammalian cell derived H5N1 HA1 or the HA0 proteins obtained from Immune Technology Corp were also analyzed. Binding was recorded using ProteOn system surface plasmon resonance biosensor instrument (BioRad Labs, Hercules, CA). (B–C) Analysis of purified H5N1 protein from Flp-In cells in SDS-PAGE under reducing conditions (B) and non-reducing conditions (C). Purified HA protein from Flp-In cell has higher order protein structure as analyzed by coomassie staining of the reducing SDS PAGE (B) and by coomassie stained non-reducing SDS PAGE (C). Subunit H5N1 vaccine (Sanofi Pasteur) was run as comparator. Western blot analysis of non-reducing SDS PAG using an anti-H5N1 HA1 antibody confirmed the identity of bands observed in coomassie stained gel in Fig. 1C.