Abstract
Citric acid cycle intermediates, including succinate and citrate, are absorbed across the apical membrane by the NaDC1 Na+/dicarboxylate cotransporter located in the kidney and small intestine. The secondary structure model of NaDC1 contains 11 transmembrane helices (TM). TM7 was shown previously to contain determinants of citrate affinity, and Arg-349 at the extracellular end of the helix is required for transport. The present study involved cysteine scanning mutagenesis of 26 amino acids in TM7 and the associated loops. All of the mutants were well expressed on the plasma membrane, but many had low or no transport activity: 6 were inactive and 7 had activity less than 25% of the parental. Three of the mutants had notable changes in functional properties. F336C had increased transport activity due to an increased Vmax for succinate. The conserved residue F339C had very low transport activity and a change in substrate selectivity. G356C in the putative extracellular loop was the only cysteine mutant that was affected by the membrane impermeant cysteine reagent, MTSET. However, direct labeling of G356C with MTSEA-biotin gave a weak signal, indicating that this residue is not readily accessible to more bulky reagents. The results suggest that the amino acids of TM7 are functionally important because their replacement by cysteine had large effects on transport activity. However, most of TM7 does not appear to be accessible to the extracellular fluid and is likely to be an outer helix in contact with the lipid bilayer.
Keywords: cysteine substitution, methanethiosulfonate reagents, site-directed mutagenesis, SLC13 family, sodium, succinate
1. Introduction
Metabolic intermediates from the citric acid cycle, including succinate, citrate and α-ketoglutarate, are transported across the plasma membrane by sodium-coupled transporters from the SLC13 family [1]. The low affinity Na+/dicarboxylate cotransporter, NaDC1, is located on the apical membrane of renal proximal tubule and small intestinal cells. NaDC1 couples the transport of three sodium ions with one divalent anion substrate [2,3]. In the kidney, NaDC1 helps regulate urinary concentrations of citrate and succinate, which may have effects on the development of kidney stones or regulation of blood pressure [4,5]. NaDC1 also participates in organic anion secretion by contributing dicarboxylates to the organic anion exchangers [6].
The secondary structure of NaDC1 is predicted to have 11 transmembrane helices (TM), with an intracellular amino terminus and an extracellular carboxy terminus [7] (Fig. 1A). The carboxy terminus contains the conserved N-glycosylation site at Asn-578 [8]. Two prolines at each end of TM7 have been shown to affect transport activity; Pro-327 appears to be important for protein function whereas Pro-351 is important for trafficking to the plasma membrane [9]. TM7 also contains residues that determine differences in citrate and sodium Km between the rabbit and human NaDC1 [10]. Finally, Arg-349 at the extracellular surface of TM7 is important for substrate and cation binding [11].
Fig. 1.
A. Secondary structure model of NaDC1. The numbered rectangles represent TMs and the Y represents N-glycosylation. The inside of the cell is at the bottom of the figure. The model indicates the positions of Pro-327, Arg-349 and Pro-351 in TM7, shown in our previous studies to be important for function [12,13] B. Multiple sequence alignment of TM7 and connecting loops in members of the SLC13 family. The amino acid numbering of the TM7 sequence alignment (321–357, shown above the sequences) is based on the rbNaDC1 sequence. The Genbank accession numbers of the nucleotide sequences are shown next to the names. The amino acids mutated in this study (321–343, 355–357) are shown in white lettering on a black background, and conserved amino acids are shown with grey backgrounds. The line below the sequences indicates the approximate position of transmembrane helix 7 and the orientation is inside (left) to outside (right).
Our previous study involved a cysteine scan at the extracellular surface of TM7, between Leu-344 to Phe-354, including Arg-349 [12]. In the present study we extended the cysteine scan to include the rest of TM7 and the associated loops, from Gln-321 to Trp-357. Despite high expression on the plasma membrane, many of the cysteine mutants had low activity or were inactive. Some of the TM7 mutants had changes in functional properties: F339C had altered succinate:citrate substrate selectivity and F336C had an increased substrate Vmax. Only one of the 26 mutants, G356C, predicted to be in the extracellular loop, was inhibited by chemical labeling with membrane impermeant cysteine selective reagents. The results suggest that the amino acids of TM7 are functionally important, because their replacement by cysteine had large effects on transport activity. However, TM7 does not appear to be accessible to the extracellular solvent and it is likely to be an outer helix.
2. Methods
2.1 Site-directed mutagenesis
Twenty-six acids in transmembrane helix (TM) 7 and associated loops of rbNaDC1 were individually mutated to cysteines using the QuikChange site-directed mutagenesis kit (Stratagene). The mutants were made in a background of 4N, a reduced cysteine mutant of NaDC1 containing containing four out of eleven endogenous cysteines at the N-terminus (positions 4, 38, 50 and 64), in the pcDNA3.1 vector (Invitrogen). NaDC1 requires at least four cysteines for expression [13] and the 4N mutant is not sensitive to membrane impermeant methanethiosulfonate reagents, such as MTSET.
2.2 Expression of NaDC1 cysteine mutants in COS-7 cells
Mutants were expressed in COS-7 cells, as described previously [14]. Briefly, the cells were cultured in Dulbecco’s Modified Eagle’s Medium containing Glutamax and 25 mM HEPES (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin and 100 g /ml streptomycin at 37°C in 5% CO2. Cells were plated on collagen-coated plates at 0.6 × 105 cells per well (24 wells) or 1.5 × 105 cells/well (6-wells) and transfected using Fugene 6 (Roche) at a 9:3 ratio (1.8 l Fugene 6 and 0.6 g plasmid DNA) for 24-well plates or a 3:1 ratio (3 l Fugene 6 and 1 g plasmid DNA) for 6-well plates.
2.3 Transport assays
Transport assays were carried out 48 hr after transfections, also as described [14,15]. The sodium buffer contained in mM: 140 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, pH adjusted to 7.4 with 1 M Tris. Choline buffer contained equimolar cholineCl in place of NaCl. For the assays, each well was washed twice with choline buffer to remove medium, then incubated with sodium buffer containing [2,3-14C]-succinate (~50 mCi/mmol, ~20 µM, Moravek) for 30 minutes at room temperature. The uptake assays were stopped and radioactivity washed away with four washes of choline buffer. The wash volume was 1 ml (24-well plates) or 3 ml (6 well plates) and the transport buffer volume was 0.25 ml (24 well plates) or 1ml (6 well plates). Cells were dissolved in 1% SDS, transferred to scintillation vials and counted. For all experiments, uptakes in vector-transfected cells were subtracted from uptakes in NaDC1 plasmid-transfected cells to correct for background counts. Transport experiments involving the G356C mutant were done at 37°C to increase the signal. In NaDC1, temperature affects the Vmax but not the Km (results not shown).
Transport experiments involving methanethiosulfonate (MTS) reagents were also done as described previously [15]. Briefly, the cells were preincubated in freshly prepared [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET, Toronto Research Chemicals) or buffer alone for ten minutes at room temperature. The preincubation solution was removed and the cells washed, then the transport activity in the cells was assayed using 14C-succinate as described above. The transport activity after MTSET treatment is expressed as a percentage of the control group preincubated with buffer only.
2.3 Transport specificity ratio measurements
For transport specificity ratio (TSR) measurements, dual-label transport assays were done involving both 10 µM [3H]succinate and 20 µM [14C]citrate in sodium buffer, as described previously [16]. The transport specificity ratio was calculated using: TSR = (νsuccinate / νcitrate) × ([citrate] / [succinate]) where νsuccinate and νcitrate are the rates of transport of [3H]succinate and [14C]citrate, [citrate] and [succinate] are the concentrations of citrate and succinate .
2.4 Cell surface biotinylations
The cell surface expression of NaDC1 mutants in COS-7 cells was determined using the membrane-impermeant reagent, Sulfo-NHS-LC-biotin (Pierce), also as described previously [15]. Briefly, each well of cells in 6-well plates was washed 3x with 3 ml PBS pH 9 containing 1 mM each Ca2+ and Mg2+ (PBS/CM), then incubated 30 min at room temperature with 0.5 ml 1.5 mg/ml Sulfo-NHS-LC-biotin (Pierce) prepared in PBS-CM, followed by 20 min on ice with 3 ml cold Quench buffer (PBS/CM with 100 mM glycine). Lysis buffer (0.5 ml per well) (1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 20 mM Tris, pH 7.5 supplemented with 10 g/ml pepstatin, 10 g/ ml leupeptin, and 0.5 mM phenylmethylsulfonylfluoride) was added followed by 30 min incubation on ice with rocking. The lysed cells were pelleted and the supernatants were incubated with 50 l Immunopure Immobilized Streptavidin (Pierce) overnight at 4°C with end-over-end rotation. The next day the beads were washed and the samples were used in Western blotting. Blots were incubated with 1:1000 dilution of anti-NaDC1 antibodies, raised in rabbits against a fusion protein of NaDC1 [8], followed by 1:7500 dilution of peroxidase-conjugated anti-rabbit IgG secondary antibody (Jackson Labs). Detection was done using Pierce Supersignal West Pico chemiluminescent substrate and Image Station 4000R (Carestream Scientific).
2.5 Labeling of Cysteine Mutants with MTSEA-biotin
NaDC1 cysteine mutants were directly labeled with MTSEA-biotin as described previously [15]. Specific labeling was determined by pre-incubation with MTSET. The mutants were expressed in HeLa cells (ATCC CCL-2) because the background signal was lower. The cells were pretreated first with 5 mM dithiothreitol, followed by 1 mM MTSET in sodium transport buffer or sodium buffer alone for 10 min at room temperature. Cells were washed 3 times with 3 ml of PBS/CM, pH 7.5, then treated with 0.5 ml of 0.1 mM N-biotinylaminoethyl methanethiosulfonate (MTSEA-biotin) (Toronto Research Chemicals) in PBS/CM, pH 7.5 for 10 min. The MTSEA-biotin was prepared as a 200 mM stock solution in dimethylsulfoxide. After the incubation, wells were washed 3 times with 3 ml of PBS/CM, pH 7.5. Lysis buffer with protease inhibitors was added to the plates which were kept on ice with rocking for cell lysis. The remaining steps were identical to those of the cell surface biotinylation procedure. Biotinylated proteins were identified by Western blotting with anti-NaDC1 antibodies.
2.6 Statistics
Duplicate or quadruplicate measurements were made for each data point. The experiments were repeated with at least three different batches of transfected cells from different passage numbers. Significant differences between groups were identified by Student’s t-test or ANOVA with P<0.05. Data are reported as means ± SEM.
3. Results
3.1 Cysteine scanning mutagenesis of TM7 and associated loops
Twenty six amino acids from Gln-321 to Trp-357, a region that includes transmembrane helix (TM) 7, were individually mutated to cysteine. Amino acids Leu-344 through Phe-354 were not included because they were tested in a previous cysteine scan [12]. The parental transporter for this study was the reduced-cysteine 4N mutant of NaDC1, containing four out of the eleven endogenous cysteines [13]. At least four cysteines are required for correct processing of NaDC1 to the plasma membrane, and the 4N mutant is insensitive to inhibition by membrane impermeant methanethiosulfonate reagents. The sequence alignment of the TM7 region from NaDC1 and other mammalian members of the SLC13 family is shown in Fig. 1B.
3.2 Cell surface expression of TM7 mutants
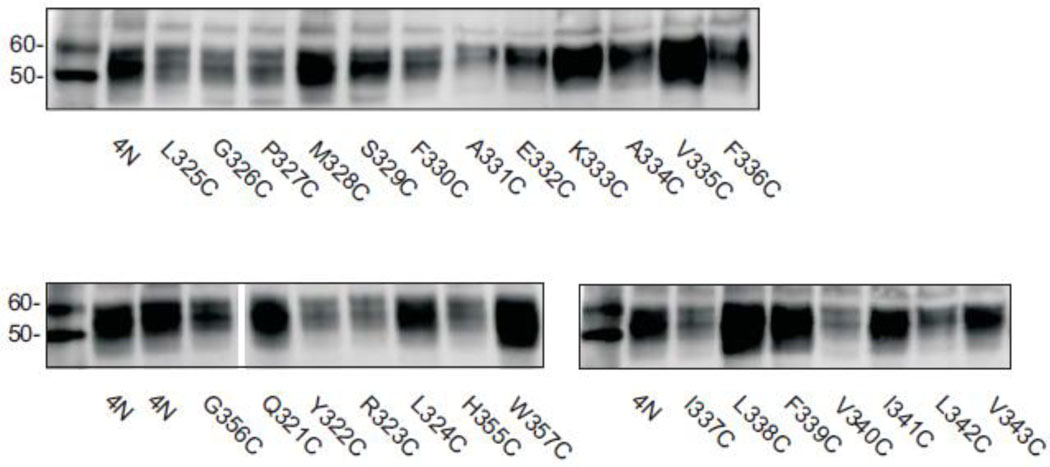
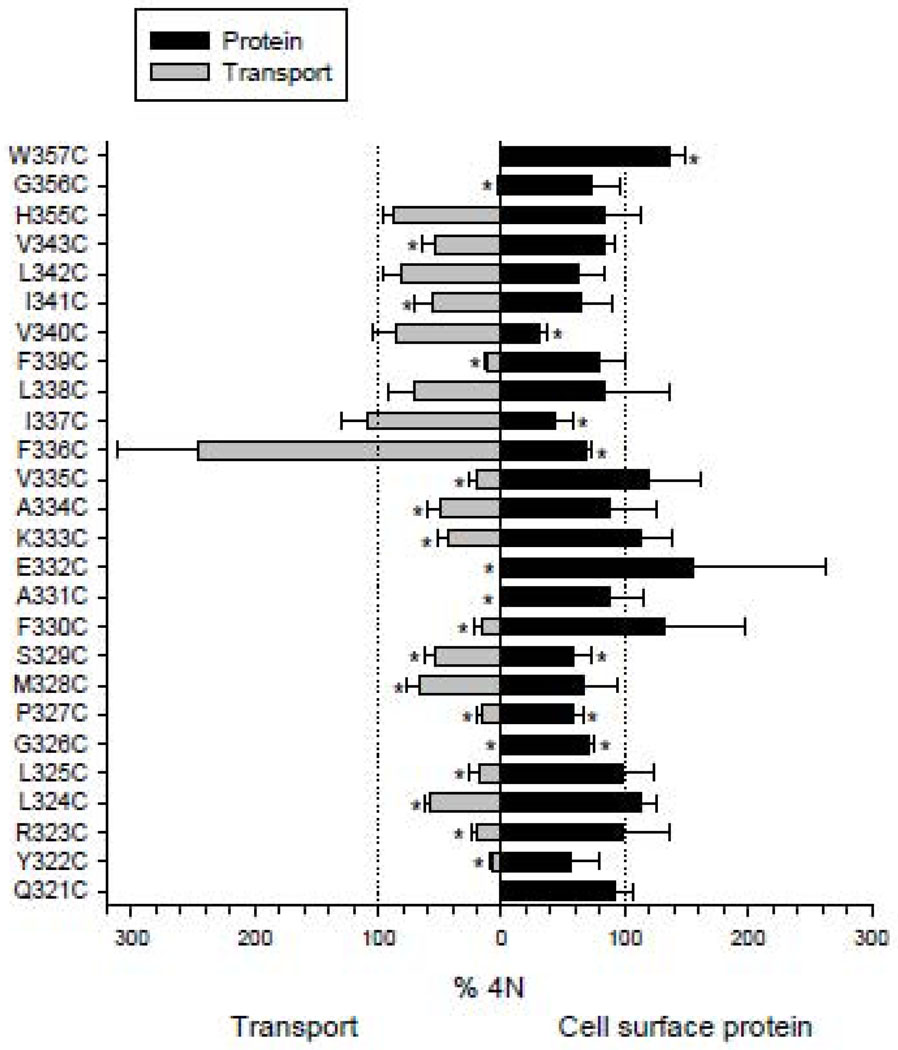
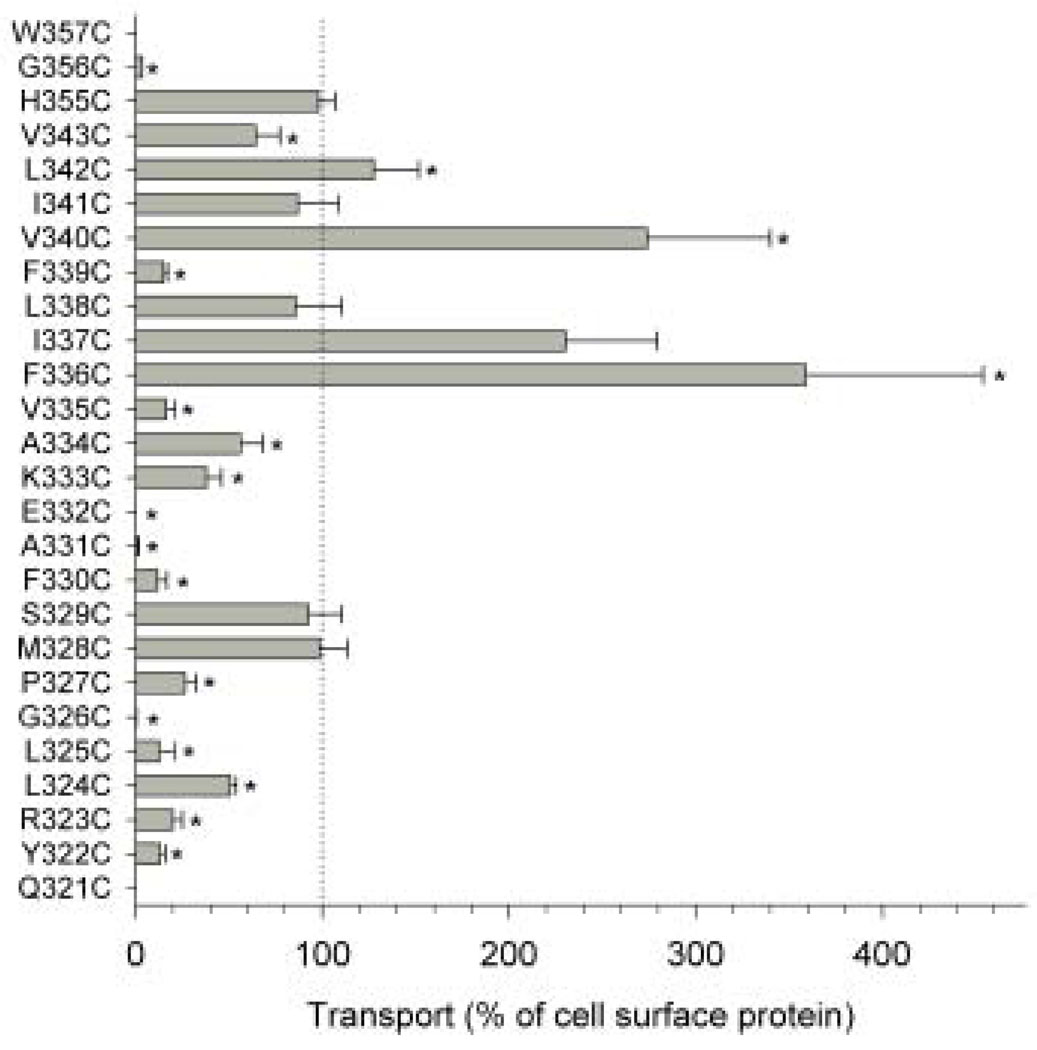
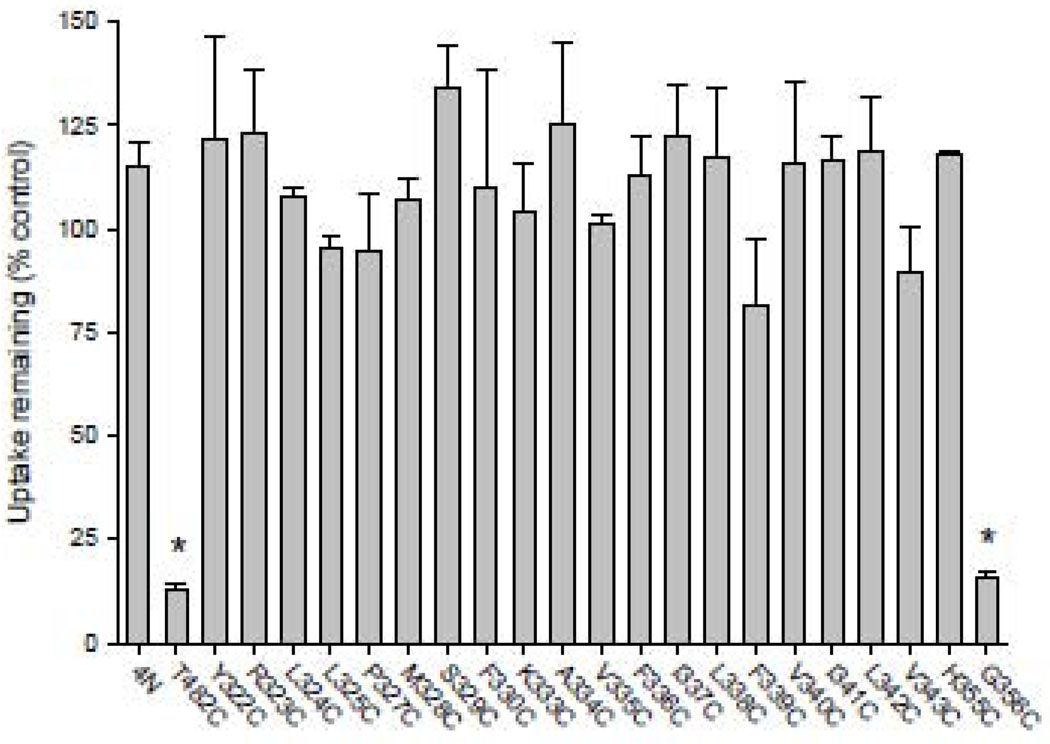
Western blots of TM7 cysteine mutants are shown in Fig. 2. COS-7 cells expressing the mutants were treated with a membrane impermeant reagent, Sulfo-NHS-LC-biotin, that labels extracellular lysine residues. All of the biotinylated cell surface proteins were then precipitated with streptavidin beads, separated on SDS-PAGE and transferred to nitrocellulose. Anti-NaDC1 antibodies were then used to identify the biotinylated NaDC1 mutants located on the plasma membrane. Images of single blots are shown in Fig. 2 and the summary of 3–4 blots is shown in Fig. 3 (right side). The succinate transport activity of the cysteine mutants is also shown in Fig. 3 (left side). Most of the mutants were well expressed on the plasma membrane compared with the 4N parental transporter. Six of the mutants had significantly lower protein abundance: G326C, P327C, S329C, F336C I337C and V340C. In contrast, the W357C mutant protein abundance was higher than that of the 4N parental (Fig. 3). Despite relatively high protein expression, many of the mutants had low transport activity, including five mutants that were completely inactive: Q321C, G326C, A331C, E332C, and W357C. When expressed as a percentage of cell surface protein abundance, the transport activity of the F336C, V340C and L342C mutants was significantly greater than the 4N parental (Fig. 4). Of the mutants with measurable transport activity, the Y322C, R323C, L324C, L325C, P327C, F330C, K333C, A334C, V335C, F339C, V343C and G356C mutants had much lower activity relative to protein expression (Fig. 4).
Fig. 2. Western blots of cell surface protein expression.
COS-7 cells expressing the TM7 cysteine mutants were treated with the membrane impermeant reagent, Sulfo-NHS-LC-biotin as described in Methods. Western blots were probed with anti-NaDC1 antibodies. The positions of MagicMark size standards at 50 and 60 kDa are in the first lane of each blot. Some lanes have been removed from the image, shown by a gap after G356C.
Fig. 3. Summary of transport activity and protein expression of the TM7 cysteine mutants.
Succinate transport activity relative to the 4N control is shown on the left side (increasing toward the left) and relative cell surface protein expression is shown on the right side of the figure. [14C]-succinate transport (~20 µM) was measured for a 30 minute time period in sodium-containing buffer. Cell surface protein abundance was determined by quantitating protein signals from Western blots (such as in Fig. 2), relative to the abundance of 4N from the same blot. Data shown are means ± SEM, n=3–6 experiments (transport) except for the W357C and Q321C transport experiments which were repeated twice; n=3–4 experiments (Western blots). The dotted lines show the 100% values and * indicates significant difference from 4N (P<0.05).
Fig. 4. Succinate transport activity relative to cell surface protein abundance in the NaDC1 cysteine mutants.
The transport activity (shown in Fig. 3) was expressed as a percentage of the mean cell surface protein abundance. Data shown are means ± SEM, n=3–6 experiments except for the W357C and Q321C transport experiments which were repeated twice. The dotted lines show the 100% values and * indicate significant difference from 4N (P<0.05).
3.3 Substrate selectivity between succinate and citrate
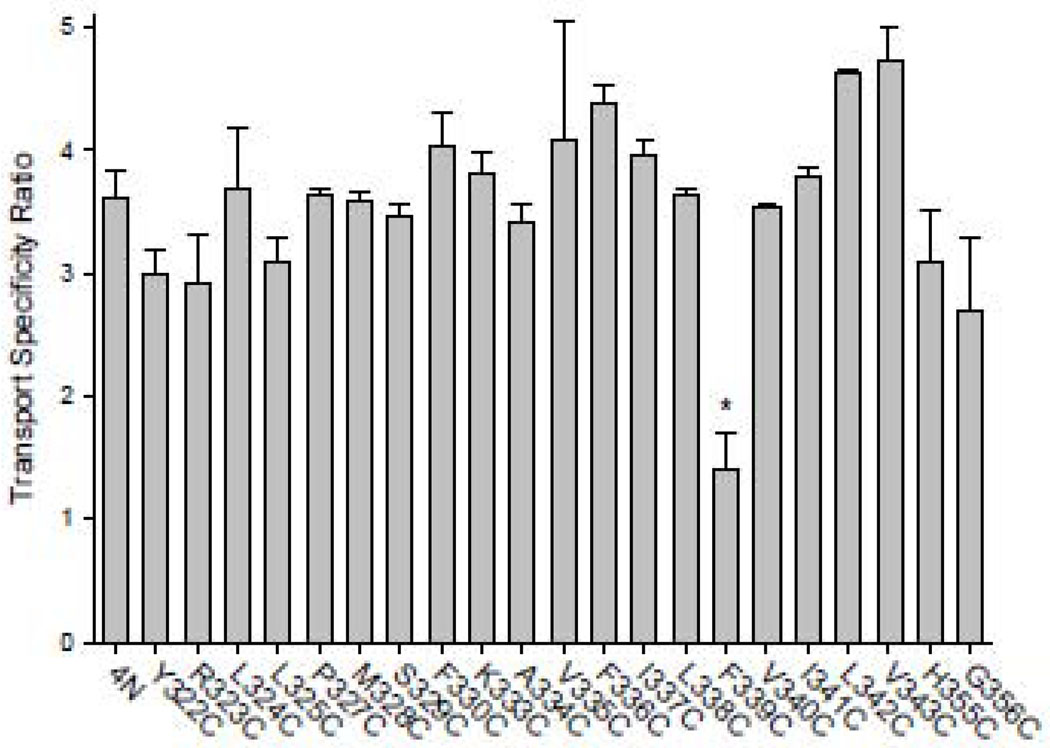
Our previous study suggested that TM7 contains determinants of citrate affinity [10]. Therefore, the transport specificity ratio (TSR) was used to identify any changes in succinate:citrate substrate selectivity in the TM7 cysteine mutants. The transport specificity ratio is independent of protein expression and is very useful for screening mutants with low transport activity [17]. As shown in Fig. 5, only the F339C mutant had a significant decrease in TSR. The other mutants, despite decreases in transport activity, had no change in the ratio. It should be noted that because the TSR is a ratio it detects changes in substrate selectivity of succinate relative to citrate, but does not identify changes in the kinetics of both substrates.
Fig. 5. Succinate:citrate Transport Specificity Ratio (TSR) in TM7 cysteine mutants of NaDC1.
Dual label competitive transport assays were conducted with 10 µM [3H]-succinate and 20 µM [14C]-citrate, as described in Methods. The data shown are means ± SEM, n=2–4 (mutants) or 10 experiments (4N). The * denotes significant difference from 4N, P<0.05.
3.4 Succinate transport kinetics
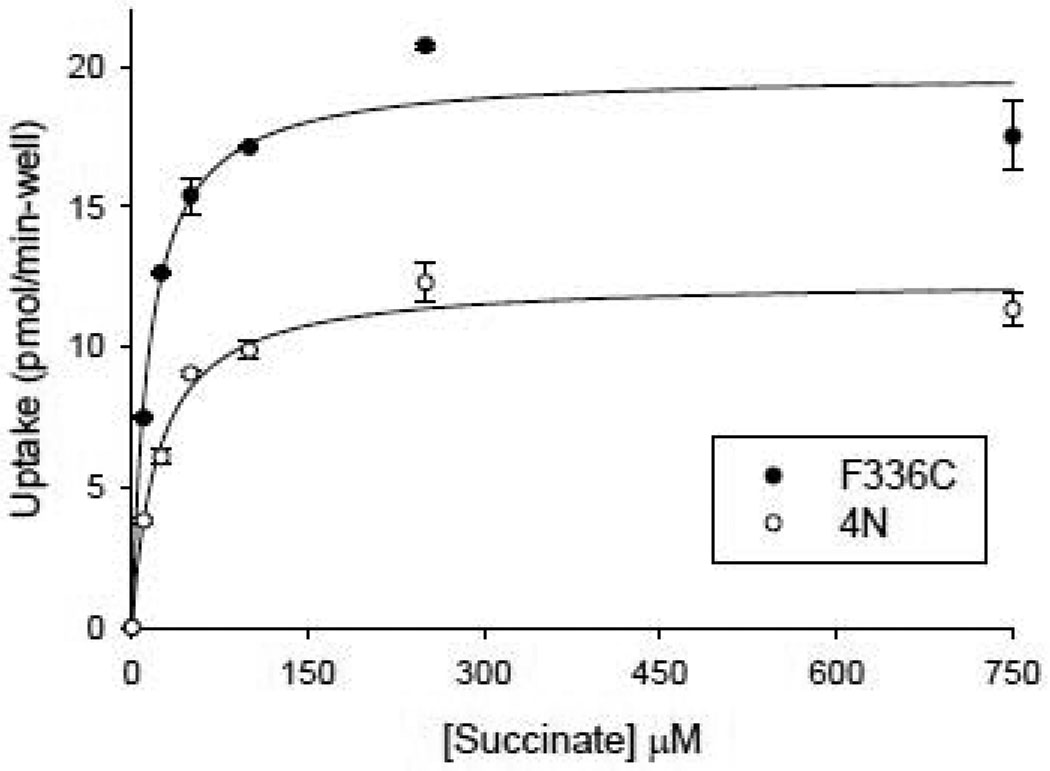
The succinate kinetics of the F336C mutant were compared with the 4N parental transporter to determine whether this could account for the increased transport activity. As shown in Fig. 6, there was a significant increase in the Vmax for succinate in F336C compared with 4N, but no significant differences in Km. In the experiment shown in Fig. 6, the 4N transporter had a succinate Km value of 23 µM and a Vmax of 12 pmol/min-well. The mean of four experiments was 18 ± 3 µM (Km) and 8 ± 2 pmol/min-well (Vmax). The F336C mutant had a Km for succinate of 15 µM (experiment shown in Fig. 6), and a mean Km of 17 ± 1 µM (n=3). The Vmax for succinate in F336C was 20 pmol/min-well (Fig. 6) and a mean Vmax of 19 ± 2 pmol/min-well. It should be noted that the succinate Km in NaDC1 varies between experiments, for unknown reasons. The values reported here are similar to the Km of 25 µM in our previous experiments using COS-7 cells [18]. However, in other experiments, also using COS-7 cells, the Km was around 100 µM [19].
Fig. 6. Succinate kinetics in F336C mutant compared with 4N.
The transport of [3H]-succinate combined with increasing concentrations of non-radiolabeled succinate was measured in the same batch of COS-7 cells expressing the parental transporter 4N or the F336C mutant. Each data point is the mean ± SEM, n=4 replicates from a single experiment. The succinate kinetic constants for 4N were: Km 23 µM, Vmax 12 pmol/min-well. The succinate kinetic constants for F336C were: Km 15 µM, Vmax 20 pmol/min-well.
3.5 Sensitivity to MTSET
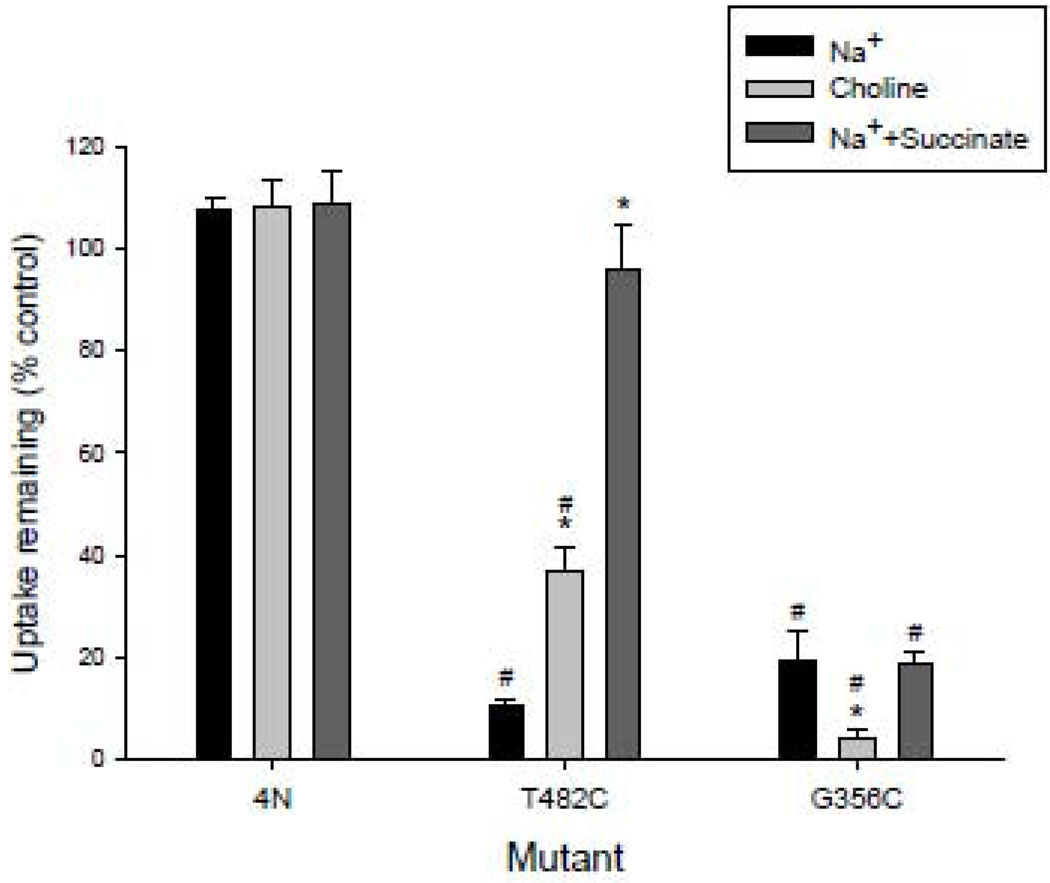
The TM7 cysteine mutants were tested for their sensitivity to the membrane impermeant reagent, MTSET. The T482C mutant was included in each experiment to verify that the MTSET was active; this mutant was shown in our previous study to be very sensitive to inhibition by MTSET [13]. Out of 26 cysteine mutants in the present study, only G356C was significantly inhibited by MTSET treatment (Fig. 7). The accessibility of G356C to extracellular MTSET was tested under different conditions, the presence or absence of sodium and sodium together with 10 mM succinate. As shown in Fig. 8, the 4N parental transporter was not inhibited by MTSET under any conditions. The T482C mutant was more strongly inhibited in the presence of sodium compared with choline, and there was substrate protection which completely prevented inhibition by MTSET. The G356C mutant was inhibited by MTSET under all conditions, but there was more inhibition in the absence of sodium. There was no evidence of substrate protection of MTSET labeling in G356C.
Fig. 7. Succinate transport activity remaining after treatment with MTSET.
COS-7 cells expressing TM7 mutants were pretreated 10 min with 1 mM MTSET in sodium buffer or sodium buffer alone (control). The succinate transport activity remaining after the MTSET pretreatment was measured using 20 µM [14C]-succinate. The succinate transport activity was expressed as a percentage of control samples treated with buffer only. The data shown are means ± SEM, n=3–4 (mutants), 8 (T482C) or 13 experiments (4N). The * denotes significant difference from 4N, P<0.05.
Fig. 8. Effect of preincubation conditions on accessibility to MTSET.
COS-7 cells expressing 4N, T482C or G356C were pretreated for 10 min with 1 mM MTSET. The MTSET pretreatment was done in sodium buffer (as in Fig. 7), choline buffer, or sodium buffer with 10 mM succinate. Control groups for each mutant were incubated in buffer only. The succinate transport activity remaining after the MTSET pretreatment was measured using 20 µM [14C]-succinate. The data shown are means ± SEM, n=3 experiments. The * indicates significant differences compared with the Na+ treatment within a group and the # indicates significant differences from the 4N group incubated under the same conditions, P<0.05.
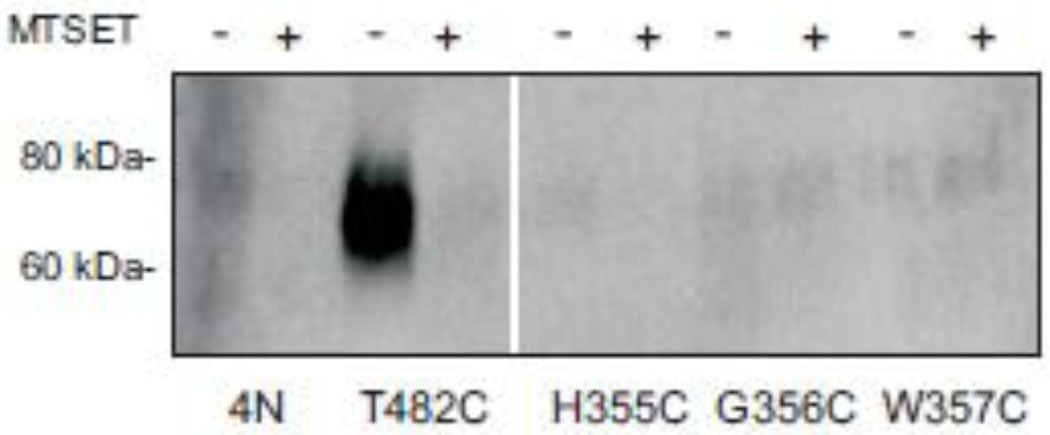
Three of the cysteine mutants in the putative extracellular loop were treated with MTSEA-biotin, followed by detection of biotinylated proteins by Western blotting. As shown in Fig. 9, the positive control mutant, T482C, was strongly labeled by MTSEA-biotin and the labeling was prevented by preincubation with MTSET. However, there was little or no signal in H355C, G356C or W357C, indicating that these mutants are difficult to label from the outside of the cell. Although low protein expression could account for low MTSEA-biotin labeling, the cell-surface protein expression of the mutants was similar to that of 4N (Fig. 2, 3). It is possible that the larger size of MTSEA-biotin accounts for the lack of labeling of G356C.
Fig. 9. Direct labeling of cysteine-substituted mutants with MTSEA-biotin.
NaDC1 cysteine mutants in the putative extracellular loop and a mutant shown previously to be sensitive to MTSET, T482C [32], were pretreated for 10 min with 1 mM MTSET (+) or sodium buffer (−) followed by chemical labeling with MTSEA-biotin. The biotinylated samples were transferred to Western blots as described in “Experimental Procedures”. The positions of size standards 60 and 80 kDa are shown. Some lanes have been removed from the image, shown by a gap. The blot was probed with anti-NaDC1 polyclonal antibodies at 1:1000 dilution.
4. Discussion
The current secondary structure model of NaDC1 contains 11 TM with an intracellular amino terminus and an extracellular N-glycosylated carboxyl terminus [7]. At present there are no high resolution crystal structures for any member of the DASS family [20] that includes the mammalian SLC13 transporters, and NaDC1. Therefore, much of our knowledge about the structure and function of the SLC13 transporters comes from mutagenesis studies. Our previous study involved a cysteine scan of ten amino acids at the top of TM7 [12]; the present study was a continuation of the cysteine scan in both directions. TM7 was chosen because several of our previous studies showed that it contains determinants of substrate affinity [10,11].
Out of the 26 amino acids mutated to cysteine in the present study, 25 were insensitive to membrane-impermeant MTS reagents. Therefore, the TM7 region of NaDC1 is likely to be an outer helix in at least partial contact with the lipid bilayer. A lack of reactivity with membrane-impermeant MTS reagents appears to be a feature of outer helices of other transporters. Cysteine scans of the lactose permease identified four helices with few or no residues that react with cysteine-specific reagents [21]. These helices were later shown in the high resolution structure to be outer helices [22]. Cysteine mutagenesis studies in the GLUT1 glucose transporter show that TM6 is a functionally-important outer helix that contains only one residue that reacts with cysteine-specific reagents [23]. In contrast, TM12 is an outer helix that provides stability to the structure of the GLUT1 transporter without being involved in the function [24]
The G356C mutant of NaDC1 was inhibited by treatment with the membrane impermeant reagent, MTSET. Gly-356 is conserved in all members of the family (Fig. 1B). Replacement of Gly-356 by Cys reduced the transport activity considerably, and chemical labeling with MTSET inhibited further. The adjacent residue in the sequence, Trp-357, is also conserved in all members of the SLC13 family and the W357C mutant was inactive despite high protein expression on the plasma membrane. These residues are located in a predicted extracellular loop (Fig. 10), although this loop does not appear to be readily accessible to more bulky reagents such as MTSEA-biotin. It is possible that the loop region is required for flexibility of the transporter during the conformational changes in the transport cycle. Glycine-301 in a loop region of the Na+/betaine symporter is thought to be important for the flexibility of the loop, and may act as a pivot point during conformational changes in the protein [25]. Glycine residues have also been shown to act as hinges in helices of ion channels and transporters [26,27] [28].
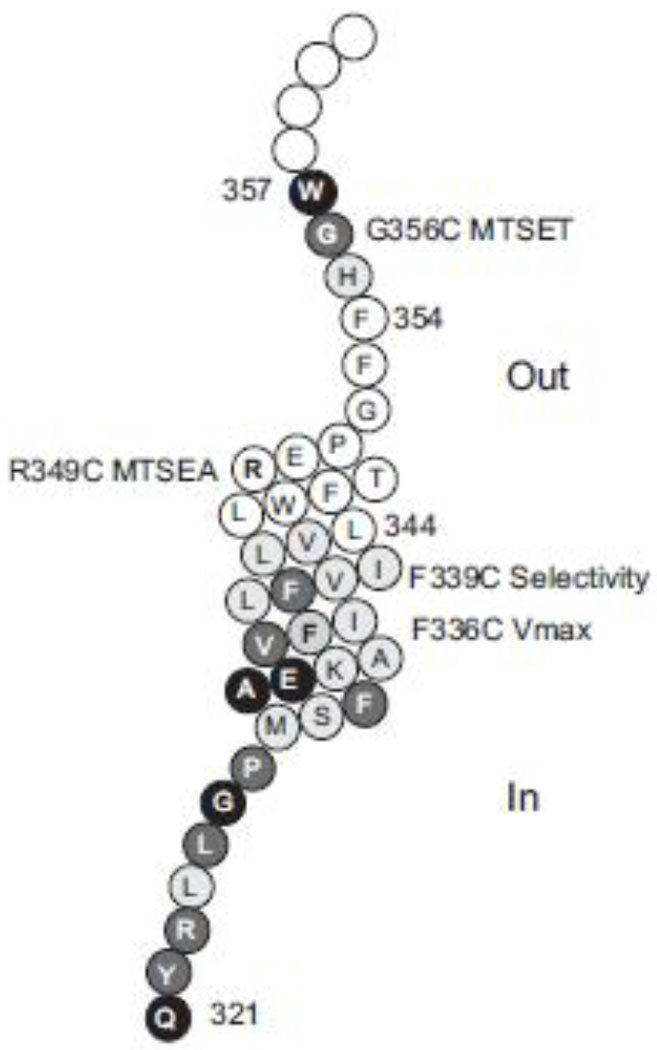
Fig. 10. Model showing functionally important residues in TM7 and associated loops.
The outside of the cell is at the top. Each amino acid is represented by a circle with the single letter amino acid abbreviation. Amino acids mutated in the present study have a grey or black background, and amino acids 344–354 mutated in our previous scan have a white background [12]. Cysteine mutants that produced inactive transporters are shown by a black background, and the mutants with low transport activity have a dark grey background, both with white lettering. The location of G356C (sensitive to MTSET), R349C (sensitive to MTSEA), F339C (altered substrate selectivity) and F336C (altered Vmax) are indicated by labels.
Arg-349 at the outer surface of TM7 was identified in our previous study as important for function [11,29]. The R349C mutant had decreased activity. Although this mutant was not sensitive to MTSET, the activity of the mutant could be restored by chemical modification with MTSEA. Cysteine modification by MTSEA is very similar in volume and charge to an arginine substitution [30]. Arg-349 is accessible to the outside of the cell in the conformational state seen in the presence of sodium, because there was no effect of MTSEA unless sodium was present. In the present study, G356C was inhibited by MTSET treatment in both sodium and choline, but the effect was greater in choline. Therefore, Gly-356 may be more accessible to the outside in the absence rather than the presence of sodium. The results of the current and previous studies with TM7 suggest that the outer portion of the helix and the loop are accessible to the outside, but the rest of the helix may be inaccessible to the extracellular solvent.
Only one of the cysteine mutants, F336C, had increased transport activity relative to that of the 4N parental transporter. This was found to be due to an increased Vmax value that was approximately double that of the 4N transporter. The protein abundance on the plasma membrane was slightly lower in F336C, indicating that the increased Vmax is likely due to faster turnover. Phe-336 is not a conserved residue, although the high affinity NaDC3 also contains Phe at this position. As shown in the model (Fig. 10) Phe-336 is located approximately one turn of the helix below Phe-339, a conserved residue that had an altered succinate:citrate selectivity after mutation to cysteine. It is possible that this face of the helix is functionally important. Unfortunately, the transport activity of F339C mutant was too low for further kinetic analysis.
The cysteine scan of TM7 revealed a surprisingly large number of mutants with little or no activity, despite good expression on the plasma membrane. Therefore, this TM appears to be more important for function than for protein targeting. This region of NaDC1 contains a large number of conserved residues (Fig. 1B), which may also indicate the functional importance of this domain. For example, Pro-327 is a conserved residue in NaDC1 and the P327C mutant also had very low transport activity. The protein does not appear to tolerate substitutions of this residue, which may be important for the structure or stability of NaDC1. Alanine or glycine substitutions of Pro-327 also resulted in very low activity and protein expression [9]. However, some conserved residues were inactive when mutated to cysteine but were able to tolerate other substitutions. For example, the E332C mutant protein was more abundant on the plasma membrane than the 4N parental but there was no transport activity. However, an alanine substitution at position 332 did not change succinate kinetics [31], indicating that the lack of activity in the E332C mutant is not due to charge neutralization. The adjacent residue in the sequence, Lys-333, is located approximately 100° away in the helix (Fig. 8). This residue is conserved in the NaDC1 orthologs, but Ile or Gln can be found at this position in other members of the family. The K333C mutant was also expressed on the plasma membrane but had decreased activity. In contrast, a previous study showed that alanine is tolerated at this postion; the K333A mutant had a similar Km to the wild-type but an increased Vmax [11].
In conclusion, this study focused on TM7 of NaDC1, a highly conserved region of the protein that contains determinants of substrate and sodium binding affinity [10,11], as well as the conformationally-sensitive residue Arg-349 [12]. The results of the present study showed that Gly-356 is functionally important and accessible to the outside of the cell, but the rest of TM7 appears to be inaccessible to the extracellular solvent. Many of the TM7 cysteine mutants had high protein expression on the plasma membrane but low or no activity, with the exception of F339C which resulted in increased transport Vmax. TM7 is likely to be an outer helix that does not directly form the substrate permeation pathway but contains amino acids that are important for function.
Research highlights
TM7 of the Na+/dicarboxylate cotransporter 1 is likely to be an outer helix
Gly-356 was inhibited by MTSET but could not be labeled by the bulkier MTSEA-biotin
Phe-336 and Phe-339 are important for substrate turnover or selectivity
TM7 contains functionally important residues; cysteine mutants had reduced activity independent of protein expression.
Acknowledgments
This work was supported by National Institutes of Health Grant DK46269 (to AMP). We thank Alva Leung and Jeffrey Young for help in making solutions.
Abbreviations
- DASS
divalent anion sodium symporter family
- MTSEA-biotin
N-biotinaminoethyl methanethiosulfonate
- MTSET
[2-(trimethylammonium)ethyl]-methanethiosulfonate
- NaDC1
sodium dicarboxylate cotransporter 1
- PMSF
phenylmethylsulfonyl fluoride
- rb
rabbit
- SLC13
solute carrier family 13
- TM
transmembrane helix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pajor AM. Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch. 2006;451:597–605. doi: 10.1007/s00424-005-1487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright SH, Hirayama B, Kaunitz JD, Kippen I, Wright EM. Kinetics of sodium succinate cotransport across renal brush-border membranes. J. Biol. Chem. 1983;258:5456–5462. [PubMed] [Google Scholar]
- 3.Pajor AM, Hirayama BA, Loo DDF. Sodium and lithium interactions with the Na+/dicarboxylate cotransporter. J. Biol. Chem. 1998;273:18923–18929. doi: 10.1074/jbc.273.30.18923. [DOI] [PubMed] [Google Scholar]
- 4.Nicar MJ, Skurla C, Sakhaee K, Pak CYC. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8–14. doi: 10.1016/0090-4295(83)90113-9. [DOI] [PubMed] [Google Scholar]
- 5.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J. Am. Soc. Nephrol. 2009;20:1002–1011. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantzler WH, Evans KK. Effect of αKG in lumen on PAH transport by isolated perfused proximal tubules. Am. J. Physiol. 1996;271:F521–F526. doi: 10.1152/ajprenal.1996.271.3.F521. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FF, Pajor AM. Topology of the Na+/dicarboxylate cotransporter: The N-terminus and hydrophilic loop 4 are located intracellularly. Biochim. Biophys. Acta. 2001;1511:80–89. doi: 10.1016/s0005-2736(00)00385-0. [DOI] [PubMed] [Google Scholar]
- 8.Pajor AM, Sun N. Characterization of the rabbit renal Na+/dicarboxylate cotransporter using anti-fusion protein antibodies. Am. J. Physiol Cell Physiol. 1996;271:C1808–C1816. doi: 10.1152/ajpcell.1996.271.6.C1808. [DOI] [PubMed] [Google Scholar]
- 9.Joshi AD, Pajor AM. Role of conserved prolines in the structure and function of the Na+/dicarboxylate cotransporter 1, NaDC1. Biochemistry. 2006;45:4231–4239. doi: 10.1021/bi052064y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn ES, Pajor AM. Determinants of substrate and cation affinities in the Na+/dicarboxylate cotransporter. Biochemistry. 1999;38:6151–6156. doi: 10.1021/bi9827722. [DOI] [PubMed] [Google Scholar]
- 11.Pajor AM, Kahn ES, Gangula R. Role of cationic amino acids in the Na/dicarboxylate co-transporter, NaDC-1. Biochem. J. 2000;350:677–683. [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X, Pajor AM. Arginine-349 and aspartate-373 of the Na+/dicarboxylate cotransporter are conformationally sensitive residues. Biochemistry. 2002:1083–1090. doi: 10.1021/bi0156761. [DOI] [PubMed] [Google Scholar]
- 13.Pajor AM, Krajewski SJ, Sun N, Gangula R. Cysteine residues in the Na+/dicarboxylate cotransporter, NaDC-1. Biochem J. 1999;344:205–209. [PMC free article] [PubMed] [Google Scholar]
- 14.Pajor AM, Randolph KM, Kerner SA, Smith CD. Inhibitor binding in the human renal low- and high-affinity Na+/glucose cotransporters. J. Pharmacol. Exp. Ther. 2008;324:985–991. doi: 10.1124/jpet.107.129825. [DOI] [PubMed] [Google Scholar]
- 15.Pajor AM, Randolph KM. Conformationally sensitive residues in extracellular loop 5 of the Na+/dicarboxylate co-transporter. J. Biol. Chem. 2005;280:18728–18735. doi: 10.1074/jbc.M501265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshiro N, King SC, Pajor AM. Transmembrane helices 3 and 4 are involved in substrate recognition by the Na+/dicarboxylate cotransporter, NaDC1. Biochemistry. 2006;45:2302–2310. doi: 10.1021/bi052328g. [DOI] [PubMed] [Google Scholar]
- 17.King SC. The "Transport Specificity Ratio": a structure-function tool to search the protein fold for loci that control transition state stability in membrane transport catalysis. BMC. Biochem. 2004;5:16. doi: 10.1186/1471-2091-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerachayaphorn J, Pajor AM. Sodium-dependent extracellular accessibility of Lys-84 in the sodium/dicarboxylate cotransporter. J. Biol. Chem. 2007;282:20213–20220. doi: 10.1074/jbc.M701113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weerachayaphorn J, Pajor AM. Threonine-509 is a determinant of apparent affinity for both substrate and cations in the human Na+/dicarboxylate cotransporter. Biochemistry. 2008;47:1087–1093. doi: 10.1021/bi701417h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saier MH. A functional phylogenetic system for the classification of transport proteins. J. Cell Biochem. 1999;32–33 Suppl:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Frillingos S, Sahin-Toth M, Wu J, Kaback HR. Cys-scanning mutagenesis: a novel approach to structure-function relationships in polytopic membrane proteins. FASEB J. 1998;12:1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 22.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and Mechanism of the Lactose Permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 23.Mueckler M, Makepeace C. Transmembrane segment 6 of the Glut1 glucose transporter is an outer helix and contains amino acid side chains essential for transport activity. J. Biol. Chem. 2008;283:11550–11555. doi: 10.1074/jbc.M708896200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueckler M, Makepeace C. Transmembrane segment 12 of the Glut1 glucose transporter is an outer helix and is not directly involved in the transport mechanism. J. Biol. Chem. 2006;281:36993–36998. doi: 10.1074/jbc.M608158200. [DOI] [PubMed] [Google Scholar]
- 25.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 26.Hussainzada N, Khandewal A, Swaan PW. Conformational flexibility of helix VI is essential for substrate permeation of the human apical sodium-dependent bile acid transporter. Mol. Pharmacol. 2008;73:305–313. doi: 10.1124/mol.107.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Digby HR, Roberts JA, Sutcliffe MJ, Evans RJ. Contribution of conserved glycine residues to ATP action at human P2X1 receptors: mutagenesis indicates that the glycine at position 250 is important for channel function. J. Neurochem. 2005;95:1746–1754. doi: 10.1111/j.1471-4159.2005.03494.x. [DOI] [PubMed] [Google Scholar]
- 28.Shang L, Tucker SJ. Non-equivalent role of TM2 gating hinges in heteromeric Kir4.1/Kir5.1 potassium channels. Eur. Biophys. J. 2008;37:165–171. doi: 10.1007/s00249-007-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao X, Pajor AM. The transport properties of the human renal Na+/dicarboxylate cotransporter under voltage clamp conditions. Am. J. Physiol. (Renal Fluid Electrolyte Physiol.) 2000;279:F54–F64. doi: 10.1152/ajprenal.2000.279.1.F54. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Kakhniashvili DA, Gremse DA, Wood DO, Mayor JA, Walters DE, Kaplan RS. The yeast mitochondrial citrate transport protein. Probing the roles of cysteines, Arg(181), and Arg(189) in transporter function. J. Biol. Chem. 2000;275:7117–7124. doi: 10.1074/jbc.275.10.7117. [DOI] [PubMed] [Google Scholar]
- 31.Griffith DA, Pajor AM. Acidic residues involved in cation and substrate interactions in the Na/dicarboxylate cotransporter, NaDC-1. Biochemistry. 1999;38:7524–7531. doi: 10.1021/bi990076b. [DOI] [PubMed] [Google Scholar]
- 32.Pajor AM. Conformationally-sensitive residues in transmembrane domain 9 of the Na+/dicarboxylate cotransporter. J. Biol. Chem. 2001;276:29961–29968. doi: 10.1074/jbc.M011387200. [DOI] [PubMed] [Google Scholar]