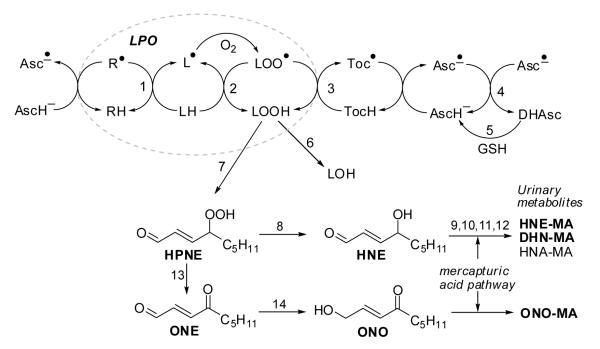
Fig. 1. Lipid peroxidation (LPO) and fate of LPO products.
The LPO chain reaction can be initiated by many radical species (indicated by R•) and converts LH into LOO•, which attacks another LH generating L• (paths 1 and 2, dotted oval). Ascorbic acid may scavenge the initiating radical species R• and reduce the tocopheroxyl radical, generating the ascorbyl radical, which can be reduced by glutathione dependent enzymes.
Key to reaction steps: 1, initiating event; 2, radical propagation reaction; 3, termination of the radical reaction by tocopherol (TocH); 4, dismutation of ascorbyl radicals (Asc•−); 5, reduction of dehydroascorbate (DHAsc) by GSH-dependent dehydroascorbate reductase; 6, GSH peroxidase (GPx); 7, further oxygenation and non-enzymatic cleavage of carbon-carbon bonds; 8, reduction. Steps 9-12 represent the mercapturic acid pathway to form mercapturic acid (= N-acetyl cysteine) conjugates: 9, glutathione-S-transferase (GST); 10, γ-glutamyl transferase; 11, cysteinyl glycinase; 12, N-acetyl transferase. HNE-GSH/MA metabolites can be converted into DHN-MA and HNA-MA. Step 13, dehydration; 14, aldo-keto reductase (AKR).