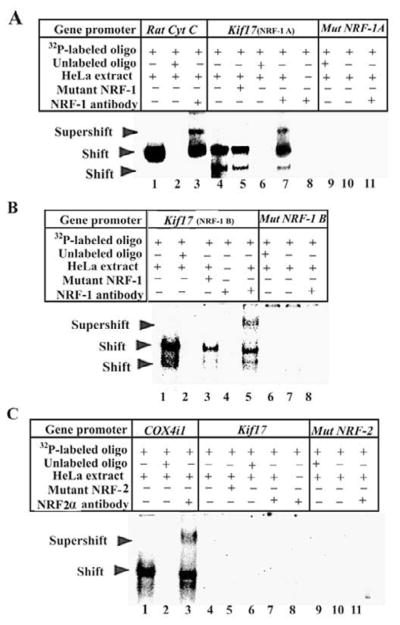
Fig. 1. NRF-1 and NRF-2α interactions in vitro with Kif17 gene promoter.
(A–B) EMSAs for NRF-1. 32P- labeled oligonucleotides, excess unlabeled oligos as competitors, excess unlabeled mutant NRF-1 as competitors, HeLa extract, and NRF-1 antibodies are indicated by a + or a – sign. Arrowheads indicate NRF-1 shift and supershift complexes. The positive control, cytochrome c, shows a shift (A, lane 1) and a supershift (A, lane 3) band. When excess unlabeled competitor was added, it did not yield any band (A, lane 2). Kif17 promoter containing two putative NRF-1 binding sites (NRF-1A and NRF-1B) showed specific shift and supershift bands that were eliminated by excess unlabeled competitors (A, lanes 4, 7, and 6; B, lanes 1, 5, and 2, respectively). Labeled mutated NRF-1 A and B sites on Kif17 were used as negative controls, and they did not yield any band (A, lanes 9–11; B, lanes 6–8, respectively). Excess unlabeled but mutated NRF-1 (containing both NRF-1A and B sites) could not compete (A, lane 5; B, lane 3, respectively). Labeled oligos with NRF-1 antibodies alone with no HeLa extract did not yield any band (A, lane 8; B, lane 4, respectively). (C) EMSA for NRF-2α. The positive control, COX4i1, shows shift (C, lane 1) and supershift (C, lane 3) bands, while excess unlabeled competitors did not yield any band (C, lane 2). Kif17 promoter had no shift or supershift bands for NRF-2α (C, lanes 4 & 7). Labeled mutated NRF-2α site did not yield any bands (C, lanes 9–11). Excess unlabeled and mutated NRF-2α sites did not show any bands (C, lane 5–6). Labeled oligos with NRF-2α antibodies alone did not yield any band (C, lane 8).