Summary
Developmental modifications in cell shape depend on dynamic interactions between the extracellular matrix and cytoskeleton. In contrast, existing models of cytokinesis describe substantial cell surface remodeling that involves many intracellular regulatory and structural proteins but includes no contribution from the extracellular matrix [1–3]. Here, we show that extracellular hemicentins assemble at the cleavage furrow of dividing cells in the C. elegans germline and in preimplantation mouse embryos. In the absence of hemicentin, cleavage furrows form but retract prior to completion, resulting in multinucleate cells. In addition to their role in tissue organization, the data indicate that hemicentins are the first secreted proteins required during mammalian development and the only known secreted proteins required for cytokinesis, with an evolutionarily conserved role in stabilizing and preventing retraction of nascent cleavage furrows. Together with studies showing that extracellular polysaccharides are required for cytokinesis in diverse species [4–9], our data suggest that assembly of a cell type-specific extracellular matrix may be a general requirement for cleavage furrow maturation and contractile ring function during cytokinesis.
Results
Hemicentin Is Required for Cleavage Furrow Stability in the C. elegans Germline
Approximately 30 distinct C. elegans him mutants produce a high incidence of male self-progeny as a result of defects in segregation of the X chromosome in the hermaphrodite germline [10]. All characterized him loci encode intracellular proteins associated with the machinery of chromosome segregation, with one exception: him-4 encodes a large secreted protein with highly conserved vertebrate orthologs, which we have named hemicentin [11]. In addition to a poorly understood defect in germline chromosome segregation, him-4 mutants exhibit pleiotropic defects in cell adhesion and migration [10, 11].
Germ cells in C. elegans have incomplete cleavage furrows that connect them to a central cytoplasmic core, allowing them to act as “nurses” while allowing more mature oocytes to fill with bulk cytoplasm [12]. Hemicentin::GFP concentrates in a ring around incomplete cleavage furrows in germ cell plasma membranes (Figure 1). In the absence of hemicentin, germ cell plasma membranes are disorganized and gonads contain multinucleate germ cells [11]. The circumferential distribution of hemicentin::GFP at the periphery of the cleavage furrow and defects in membrane organization observed in the absence of hemicentin suggest that hemicentin may be required for either the assembly or the stability of nascent membrane structures at the cleavage furrow. To distinguish between these two possibilities, we examined plasma membrane structure in a hemicentin mutant [him-4 (rh319)] background, utilizing a membrane-associated RFP-tagged phospholipase C δ PH domain (RFP-PH). Temporal studies show that recently synthesized membranes have a relatively normal structure but are unstable and retract with time, resulting in multinucleate germ cells that increase in number and frequency with maternal age (Figure 1; see also Table S1 available online). Wild-type animals of all ages and him-4(rh319) mutant animals prior to and early in adulthood have few multinucleate cells. In contrast, older him-4(rh319) adults (>24 hr following final molt) have large numbers (~25%) of multinucleate gametocytes (Table S1). Similar nonspecific effects in him-4(rh319) and control animals grown in a variety of osmotic conditions (Figure S1) suggest that the role of hemicentin in cytokinesis is distinct from that of several glycosaminoglycan synthesis genes that appear to promote cytokinesis by generating osmotic pressure inside the eggshell [4–7].
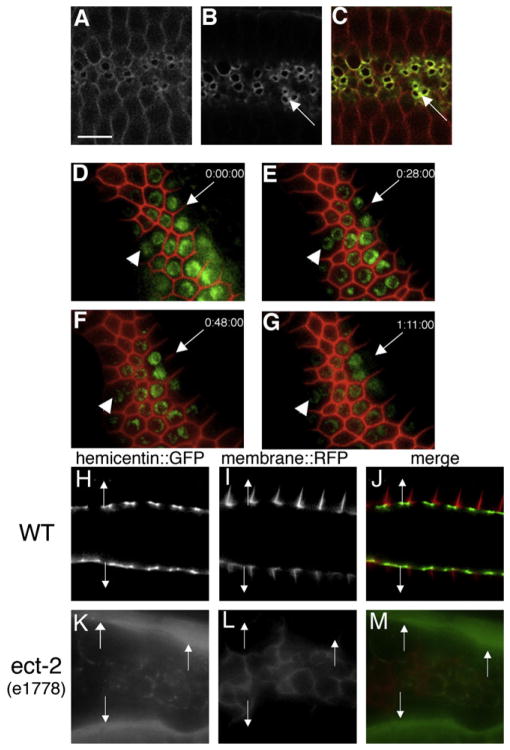
Figure 1. Hemicentin Assembles at the Cleavage Furrow of C. elegans Germ Cells and Prevents Membrane Retraction.
Germ cells in the hermaphrodite distal gonad have incomplete cleavage furrows.
(A–C) Optical section through the central region of C. elegans gonad showing wild-type C. elegans hermaphrodite germ cells coexpressing RFP::phospholipase C δ PH domain (A and C) and hemicentin::GFP (B and C). Note that hemicentin::GFP accumulates at the periphery of incomplete cleavage furrows (arrows).
(D–G) Progressive retraction of germ cell membranes in the absence of hemicentin. Images show a time course of a single him-4(rh319) hermaphrodite mutant gonad expressing RFP:PH and histone 2B:GFP transgenes. Images were collected at 0 (D), 28 (E), 48 (F), and 71 min (G). Note progressive retraction of the germ cell membrane (arrow). Arrowhead indicates one of several multinucleate germ cells present prior to image collection. This effect does not depend on osmotic pressure (Figure S1). Phenotype quantitation is presented in Table S1.
(H–M) The ECT-2 RhoGEF is required for hemicentin assembly in the gonad. Comparison of membrane RFP:PH and hemicentin:GFP distribution in a wild-type and ect-2 mutant background is shown. In a wild-type background, hemicentin:GFP assembles peripheral to incomplete cleavage furrows. In an ect-2(e1778) mutant background, cleavage furrows do not form properly, and hemicentin does not assemble at membrane surfaces but accumulates outside the gonad (arrows). Scale bar represents 5 μm.
Hemicentin is not synthesized by germ cells but is synthesized outside the germline by somatic gonad and body wall muscle cells that secrete hemicentin into the pseudocoelomic fluid [11]. In wild-type animals, secreted hemicentin is targeted to germ cell surfaces by specific sequences within the von Willebrand A domain of hemicentin [13]. Assembly of hemicentin in the gonad is dependent on ECT-2, a highly conserved RhoGEF that activates Rho in a number of eukaryotic species to initiate contractile ring assembly and to promote cleavage furrow stability [14, 15]. In an ect-2(e1778) mutant background, only a small amount of hemicentin::GFP is recruited to germ cell surfaces inside the gonad, whereas the bulk of the hemicentin:: GFP remains in the pseudocoelomic fluid outside the gonad (Figures 1H–1M). This suggests that ECT-2 is important for the assembly of a structure in the nascent cleavage furrow of dividing germ cells that promotes the recruitment and assembly of hemicentin.
Hemicentin-1 Localizes to the Cleavage Furrow and Is Required for Cytokinesis in Preimplantation Mouse Embryos
To test whether hemicentin’s function in cleavage furrow stability is conserved in mammals, we examined the role of hemicentin-1 during mouse development. Two highly conserved hemicentin orthologs, hemicentin-1 (also known as fibulin-6) and hemicentin-2, have a pericellular distribution in a number of mouse tissues including adult epidermis and trophectoderm cell junctions in preimplantation mouse embryos [16]. Examination of hemicentin distribution with an antibody that recognizes hemicentin-1 and -2 prior to trophectoderm formation revealed a diffuse staining pattern that became punctate and concentrated at or near the presumptive cleavage furrow of mitotic one-cell mouse embryos. Hemicentins remained concentrated in the nascent furrow during the division process and formed an open ring structure peripheral to the contractile ring component myosin IIB (Figure 2). This pattern was repeated in successive cell divisions and suggests a potential role for vertebrate hemicentins in mouse blastomere cytokinesis that is analogous to the germline function of C. elegans hemicentin (Figure 2).
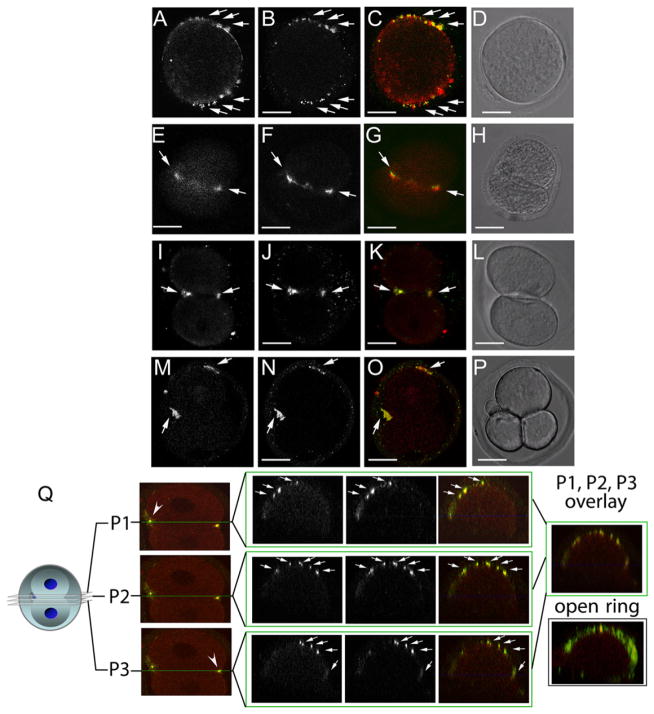
Figure 2. Hemicentin Distribution in Wild-Type Mouse Embryos.
(A–P) Immunofluorescence images of dividing one- (A–D), two- (E–L), and three-cell (M–P) wild-type embryos. Embryos were stained at multiple time points with antibodies to hemicentin (A, E, I, and M) and myosin IIB (B, F, J, and N). Merged images (C, G, K, and O) indicate that hemicentin staining accumulates adjacent to myosin II staining in dividing cells. Also shown are corresponding differential interference contrast (DIC) images (D, H, L, and P). Scale bars represent 20 μm.
(Q) Confocal image of three separate planes across the cleavage furrow of a dividing one-cell embryo stained with hemicentin (red) and myosin IIB (green) antibodies. Right: overlay of merged images from two different embryos showing that both proteins form a punctate open ring around the periphery of the cleavage furrow.
To determine whether hemicentins have an essential function at this early time during mouse embryonic development, the function of hemicentin-1 was tested by two techniques: double-stranded RNA (dsRNA)-mediated knockdown and targeted inactivation of the hemicentin-1 gene. Injection of hemicentin-1 dsRNA into one-cell wild-type mouse embryos resulted in a large number of embryos (89%) that arrested prior to the four-cell stage, compared to <4% of control embryos that arrested at this stage (Table S2). Arrested embryos had multinucleate cells that were not detected in mock-injected or E-cadherin dsRNA-injected control embryos (Figure 3).
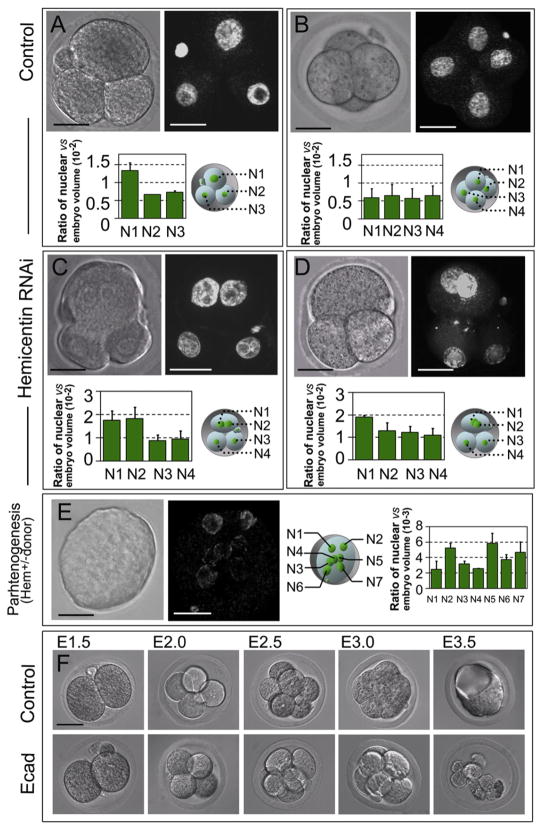
Figure 3. Hemicentin-1 Depletion Results in Embryonic Arrest Prior to Implantation.
(A–D) Wild-type embryos were mock injected (A and B) or injected at the one-cell stage with hemicentin-1 dsRNA (C and D). Control embryos progress through the three- and four-cell stage with single nuclei (A and B). In contrast, hemicentin-1 dsRNA-injected embryos arrest prior to the four-cell stage with multinucleate cells (C and D). DIC and corresponding YOYO-1-stained images are shown. Ratio of nuclear volume to embryonic volume (×10−2) with corresponding schematic diagram is shown below each embryo. Error bars represent standard deviation. Larger nuclear volumes of N1 and N2 in (C) and N1 in (D) suggest that these nuclei are in G2 phase [27].
(E) Parthenogenic activation of eggs obtained from heterozygous hemicentin-1 females results in arrest of embryos with multinucleate cells. DIC and corresponding YOYO-1-stained images of embryos obtained from parthenogenic activation of eggs collected from heterozygous females are shown. Approximately 40% of eggs divide to become mononucleate cells, and ~60% (Table S2) arrest at the one- to three-cell stage, with most cells containing multiple nuclei. At least seven distinct nuclei (N1–N7) are detected within the single arrested blastomere. Ratio of nuclear volume to embryonic volume (×10−3) with corresponding schematic diagram is shown to the right. Error bars represent standard deviation.
(F) As described above, the majority of mock-injected embryos successfully develop into blastocysts (see Table S2). As a positive control, most E-cadherin dsRNA-injected embryos proliferate normally from the two- to eight-cell stage but fail to form a blastocoele and arrest at the morula stage (Table S2), consistent with published information on the phenotype of E-cadherin knockout mice [29].
Scale bars represent 20 μm.
The function of hemicentin-1 in early embryogenesis was also tested by targeted inactivation (Figure S2). Approximately 28% (22 of 80) of embryos obtained by mating heterozygous males and females were homozygous mutant animals that arrested between the one- and four-cell stage, compared to 0% (0 of 76) of animals arrested from matings of wild-type males and females (Figure 4). Homozygous mutant embryos had multinucleate cells and often had internal polar bodies and plasma membranes with multiple invaginations (Figure 4; Figure S3). Time-lapse imaging of negative control and homozygous mutant animals showed that after the initial formation, cleavage furrows failed to ingress and often retracted in the absence of hemicentin-1 (Figure 4; Movie S1; Movie S2). The data suggest that hemicentin is required to stabilize and promote the ingression and/or prevent the retraction of the cleavage furrow in mouse embryos. The presence of internal polar bodies in hemicentin-1-deficient embryos (Figure 4) and the detection of hemicentin on polar bodies at the time of extrusion (data not shown) suggest that hemicentins may have a role in stabilization of the cleavage furrow during polar body extrusion as well as cytokinesis.
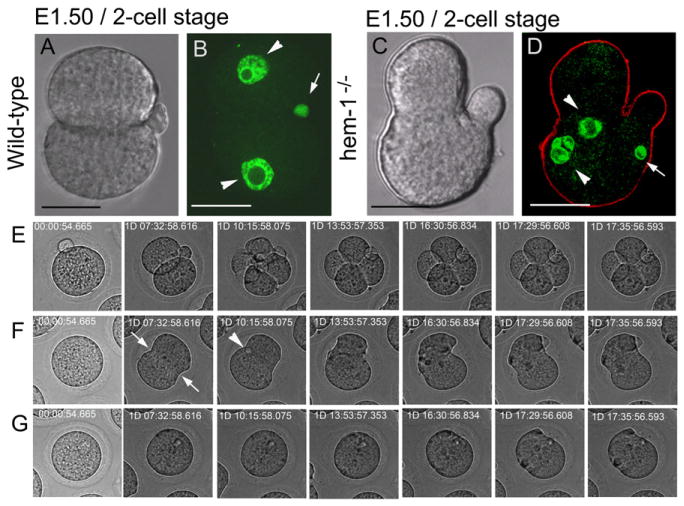
Figure 4. Hemicentin-1-Deficient Embryos Arrest Prior to Implantation with Multiple Nuclei.
(A–D) Images of age-matched wild-type (A and B) and hemicentin-1-deficient (C and D) embryos from matings of wild-type and hemicentin-1 heterozygous parents. DIC images are shown with corresponding nuclear staining with YOYO-1 (green) and anti-myosin IIB (red). Scale bars represent 20 μm.
(A and B) Wild-type embryos progress to the two-cell stage with mononucleate cells (arrowheads) and external polar bodies (arrow).
(C and D) In contrast, hemicentin-deficient embryos arrest prior to the four-cell stage. Arrested embryos contain multinucleate cells (arrowheads; Figure S3), multiple invaginations in the plasma membranes, and internal polar bodies (arrow).
(E–G) Time-lapse photography of wild-type and hemicentin-deficient embryos in Movie S1 and Movie S2 during the first 3 days of development. Wild-type embryos have external polar bodies and develop to the four-cell stage (E; Movie S1). In contrast, hemicentin-1-deficient embryos develop membrane invaginations (arrows) and internal polar bodies (arrowhead), but membrane invaginations do not proceed to completion and often retract (F and G; Movie S2).
Unfertilized eggs obtained from females heterozygous for the null allele of hemicentin-1 were also parthenogenically activated. Approximately 64% of parthenogenically activated eggs from heterozygous mothers arrested before the four-cell stage, as compared to ~25% of parthenogenotes from wild-type mothers (Table S2). It is likely that a fraction of the hmcn-1+/0 embryos from wild-type and heterozygous mothers arrest as a result of experimental manipulation or absence of the paternal genome [17, 18]. However, a substantial fraction of the arrested embryos that were hemizygous hemicentin-1 null parthenogenotes arrested with multinucleate cells that were never detected in control embryos, and many of the cells in arrested embryos had more than two nuclei (Figure 3).
Discussion
In the C. elegans germline, substantial numbers of germ cells with multiple nuclei accumulate in aging hemicentin mutant animals as a result of membrane instability. Distribution of hemicentin peripheral to the incomplete cleavage furrows of syncytial germ cells and retraction of syncytial membranes in the absence of hemicentin suggest that hemicentin has an unprecedented role among secreted proteins in stabilizing the cleavage furrow during cytokinesis in C. elegans germ cells. The presence of multinucleate cells in preimplantation mouse embryos lacking hemicentin-1 suggests that this function of hemicentins has been conserved in the vertebrate embryo. Although we suggest that the binucleate cells observed in hemicentin-1-deficient mouse embryos indicate a failure in cytokinesis, a conceivable alternate explanation is that there is a cell-cycle defect or there has been selection for embryos that are fixed just prior to cytokinesis by chance. However, the presence of hemicentin-1 knockdown and knockout embryos with three or more nuclei, including nuclei that have doubled in volume, indicates that the cell cycle remains intact in the absence of hemicentin-1. Moreover, localization of hemicentin in the cleavage furrow of dividing cells and dynamic observations of plasma membrane retraction in hemicentin-1-deficient embryos, together with the fact that multinucleate cells were never detected in control embryos, strongly suggest that the multinucleate phenotype is a result of cytokinesis failure and not a secondary consequence of a cell-cycle defect or an artifact of fixation time.
Although there are striking parallels between hemicentin function in mouse blastomeres and C. elegans germ cells, there are also several critical differences. Hemicentin-1 appears to be required for cell proliferation in mouse blastomeres, but hemicentin is not essential for germ cell proliferation in C. elegans: hemicentin mutant hermaphrodites produce large numbers of aneuploid gametes [10, 11]. This may reflect p53 activity that restricts the proliferation of aneuploid mouse blastomeres but not aneuploid C. elegans germ cells [19]. Another critical difference is that the role for hemicentin in cytokinesis appears to be restricted to the germline in C. elegans, because no defects have been detected in C. elegans somatic cell divisions in hemicentin mutant animals [11, 20]. Instead, hemicentin appears essential for the adhesion and migration of select cells in the nematode soma. It is not known whether hemicentin has a function in cytokinesis or cell adhesion in the post-implantation mouse. C. elegans germ cells and mouse blastomeres are uncommitted cell types that divide very slowly, and it is possible that the requirement for hemicentin is limited to cleavage furrows that have structural or dynamic properties not found in rapidly dividing or highly differentiated cells.
Hemicentin-1 is the only known extracellular protein with an evolutionarily conserved role in cytokinesis and may be the first secreted protein required during mammalian development. The ability to form line-shaped elastic fiber-like polymers and transient structures during cell migration [11] suggests that hemicentins may have the properties required to perform structural roles in narrow and highly dynamic groove-shaped structures such as the cleavage furrow. However, not all eukaryotic species have a gene that encodes hemicentin, indicating that hemicentins are not universally required for cytokinesis. Instead, several studies have shown that synthesis of glycosaminoglycans and other extracellular polysaccharides is essential for cleavage furrow stability and maturation in diverse species [4–9]. Together, these studies suggest that extracellular matrices may be required for cytokinesis and that there may be some specificity in the composition and organization of the extracellular matrix required in the cleavage furrow of different cell types.
Experimental Procedures
Targeted Inactivation of the Mouse Hemicentin-1 Gene
Hemicentin-1 clones were identified by hybridization using a PCR-generated probe to screen a mouse 129SvEv genomic bacterial artificial chromosome library (RPCI-22; BAC/PAC Resources, Children’s Hospital Oakland) and purchased from Invitrogen. These genomic clones were used to construct a targeting vector designed to replace the first exon of hemicentin-1 with an expression cassette containing GFP and neomycin resistance genes by homologous recombination (Figure S2).
Collection and Culture of Mouse Embryos
All female and male mice used in the experiments were 8–12 and 10–14 weeks of age, respectively, and were maintained in a 12 hr light/12 hr dark cycle. Embryos at different stages were collected from the ampulla and lumen of the uterus depending on experimental requirements. For embryo culture, single-cell oocytes were collected from the uterine ampulla and cultured in potassium-supplemented simplex optimization medium (KSOM) containing 0.5% bovine serum albumin (BSA; Sigma) under embryo test paraffin oil (Sigma) in culture dishes in an atmosphere of 5%CO2 at 37°C.
Immunocytochemistry
After collection, embryos were washed with KSOM medium, fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS), and permeabilized in PBS containing 0.25% Triton X-100 for 10 min. Immunocytochemical staining was performed by incubating the fixed samples with primary antibodies in PBS/Tween (PBS containing 0.1% Tween 20 and 3% BSA) for 1–2 hr followed by incubation with appropriate secondary antibodies diluted according to the manufacturer’s recommendation in PBS/Tween solution for 1–2 hr. Stained samples were analyzed with a Zeiss LSM 510 confocal microscope.
Double-Stranded RNA Preparation and Microinjection
Total RNA was isolated from E12.5 mouse embryos using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcriptions were performed with a SuperTranscript kit (Invitrogen) using random hexanucleotide primers and RNaseH-MMLV reverse transcriptase. Microinjection of dsRNAs was performed as described previously [21, 22].
Parthenogenic Activation of MII Phase Oocytes
Murine parthenogenesis of eggs in second meiotic metaphase was performed according to standard protocols [23–25]. Wild-type (hmcn-1+/+) and heterozygous (hmcn-1+/−) females 8–12 weeks of age were used as oocyte donors.
Embryonic/Nuclear Volume Measurements
Embryonic and nuclear volumes were calculated as described previously [26] with three distinct measurements of each zona pellucida and nuclear radius using Zeiss LSM 510 image browser software. Volume was calculated as 4/3πabc, and the ratio of nuclear to embryonic volume was used to assess cell-cycle phase. Average values and standard deviations were based on four to six independent measurements of each nucleus.
Nematode Strains
All nematode strains were cultured under standard conditions unless noted otherwise. The rhIs23 hemicentin:GFP transgene has been described previously [11]. The RFP-phospholipase Cδ1 was obtained from strain DP400 [27]. Histone H2B::GFP was obtained from strain JH1993 [28]. The ect-2(e1778) allele was obtained from Caenorhabditis Genetics Center strain CE1857.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Science Foundation (MCB-0744838) and the National Institutes of Health (GM65184). The authors would like to express their gratitude to E. Hedgecock, A. Bortvin, T. Schedl, M.-L. Chu, and R. Fassler for valuable advice during the course of this work and C.-M. Fan and M. Monteiro for comments on the manuscript. The authors would also like to thank C. Merritt, G. Seydoux, T. Kachur, A. Audhya, and D. Pilgrim for C. elegans strains; M. Karbowski, O. Jones, J. Bryant, B. Hagen, and A. Ziman for assistance with and access to microscopy and microinjection equipment; and M.-M. Xu for artwork. Some nematode strains used in this work were obtained from the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Footnotes
Supplemental Information includes three figures, two tables, Supplemental Experimental Procedures, and two movies and can be found with this article online at doi:10.1016/j.cub.2010.12.006.
References
- 1.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 2.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: From parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 3.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White J, Bednarek S. Cytokinesis: GAGs form the walls that separate our parts. Curr Biol. 2003;13:R717–R718. doi: 10.1016/j.cub.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 5.Hwang HY, Olson SK, Esko JD, Horvitz HR. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature. 2003;423:439–443. doi: 10.1038/nature01634. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Spang A, Sullivan MA, Hryhorenko J, Hagen FK. The terminal phase of cytokinesis in the Caenorhabditis elegans early embryo requires protein glycosylation. Mol Biol Cell. 2005;16:4202–4213. doi: 10.1091/mbc.E05-05-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson SK, Bishop JR, Yates JR, Oegema K, Esko JD. Identification of novel chondroitin proteoglycans in Caenorhabditis elegans: Embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol. 2006;173:985–994. doi: 10.1083/jcb.200603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishihama R, Schreiter JH, Onishi M, Vallen EA, Hanna J, Moravcevic K, Lippincott MF, Han H, Lemmon MA, Pringle JR, Bi E. Role of Inn1 and its interactions with Hof1 and Cyk3 in promoting cleavage furrow and septum formation in S. cerevisiae. J Cell Biol. 2009;185:995–1012. doi: 10.1083/jcb.200903125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumikawa T, Kanagawa N, Watamoto Y, Okada M, Saeki M, Sakano M, Sugahara K, Sugihara K, Asano M, Kitagawa H. Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J Biol Chem. 2010;285:12190–12196. doi: 10.1074/jbc.M110.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 12.Schedl T. Developmental Genetics of the Germ Line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans. II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 241–270. [PubMed] [Google Scholar]
- 13.Dong C, Muriel JM, Ramirez S, Hutter H, Hedgecock EM, Breydo L, Baskakov IV, Vogel BE. Hemicentin assembly in the extracellular matrix is mediated by distinct structural modules. J Biol Chem. 2006;281:23606–23610. doi: 10.1074/jbc.M513589200. [DOI] [PubMed] [Google Scholar]
- 14.Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–294. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory SL, Lorensuhewa N, Saint R. Signaling through the RhoGEF Pebble in Drosophila. IUBMB Life. 2010;62:290–295. doi: 10.1002/iub.310. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Dong C, Vogel BE. Hemicentins assemble on diverse epithelia in the mouse. J Histochem Cytochem. 2007;55:119–126. doi: 10.1369/jhc.6A6975.2006. [DOI] [PubMed] [Google Scholar]
- 17.McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 18.Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo JS, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–864. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- 19.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedgecock EM, Herman RK. The ncl-1 gene and genetic mosaics of Caenorhabditis elegans. Genetics. 1995;141:989–1006. doi: 10.1093/genetics/141.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonn S, Khang I, Kim K, Rhee K. Suppression of Nek2A in mouse early embryos confirms its requirement for chromosome segregation. J Cell Sci. 2004;117:5557–5566. doi: 10.1242/jcs.01476. [DOI] [PubMed] [Google Scholar]
- 22.Khang I, Sonn S, Park JH, Rhee K, Park D, Kim K. Expression of epithin in mouse preimplantation development: Its functional role in compaction. Dev Biol. 2005;281:134–144. doi: 10.1016/j.ydbio.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Balakier H, Tarkowski AK. Diploid parthenogenetic mouse embryos produced by heat-shock and Cytochalasin B. J Embryol Exp Morphol. 1976;35:25–39. [PubMed] [Google Scholar]
- 24.Robertson EJ, Evans MJ, Kaufman MH. X-chromosome instability in pluripotential stem cell lines derived from parthenogenetic embryos. J Embryol Exp Morphol. 1983;74:297–309. [PubMed] [Google Scholar]
- 25.Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, Kirby A, Daly MJ, Daley GQ. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–486. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- 26.Aiken CE, Swoboda PP, Skepper JN, Johnson MH. The direct measurement of embryogenic volume and nucleocytoplasmic ratio during mouse pre-implantation development. Reproduction. 2004;128:527–535. doi: 10.1530/rep.1.00281. [DOI] [PubMed] [Google Scholar]
- 27.Kachur TM, Audhya A, Pilgrim DB. UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C. elegans. Dev Biol. 2008;314:287–299. doi: 10.1016/j.ydbio.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino I, Merritt C, Chen PL, Seydoux G, Subramaniam K. Translational repression restricts expression of the C. elegans Nanos homolog NOS-2 to the embryonic germline. Dev Biol. 2006;292:244–252. doi: 10.1016/j.ydbio.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.