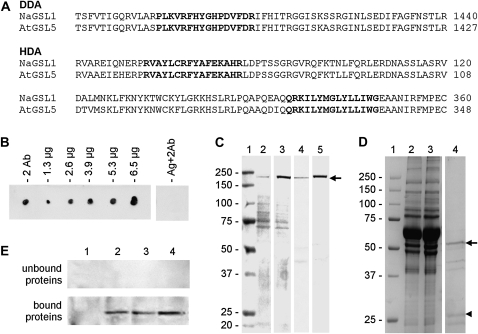
Figure 1.
Characterization of antibodies to CalS. A, Sequences of CalS used for the production of peptide antibodies. The antibody DDA was raised against a peptide sequence (in boldface) located at the C-terminal domain of CalS. The antibody HDA was raised against two peptide sequences (in boldface) found at the N-terminal domain of both N. alata (Na) and Arabidopsis (At) CalS. In both cases, numbers on the right indicate the positions of sequences in the protein. B, Dot blot showing the characterization of HDA; the antibody was assayed without antigen (2 Ab) on 1.3, 2.6, 3.9, 5.3, and 6.6 μg of antigen peptides conjugated to KLH; as a control, the antigen peptide was also tested with the secondary antibody only (Ag+2Ab). C, Immunoblot showing the screening of HDA antibody on cytosolic proteins from tobacco pollen tubes (lane 2), membrane proteins (lane 3), and cell wall proteins (lane 4) from tobacco pollen tubes in addition to extract from Arabidopsis flowers (lane 5); a polypeptide of 225 kD is prominently recognized in lanes 3 to 5. About 30 μg of protein was loaded in each lane. Prestained Mr markers were loaded in lane 1. All lanes are from the same blot. D, SDS-PAGE showing the partial purification of HDA antibody by absorption of nitrocellulose membranes coated with antigen peptides. Lane 1, Markers of Mr; lane 2, starting antiserum; lane 3, unbound proteins; lane 4, absorbed proteins released by acid solution (purified antibody). Arrow and arrowhead indicate the presumptive heavy and light chains of HDA antibody, respectively. E, Cross-reactivity of antigen-unbound proteins (sample in lane 3 of D) and of antigen-bound proteins (the purified antibody, lane 4 of D) against cytosolic proteins (lane 1), membrane proteins (lane 2), and cell wall proteins (lane 3) from pollen tubes and against extracts of Arabidopsis flowers (lane 4). Lanes contain 30 μg of proteins.