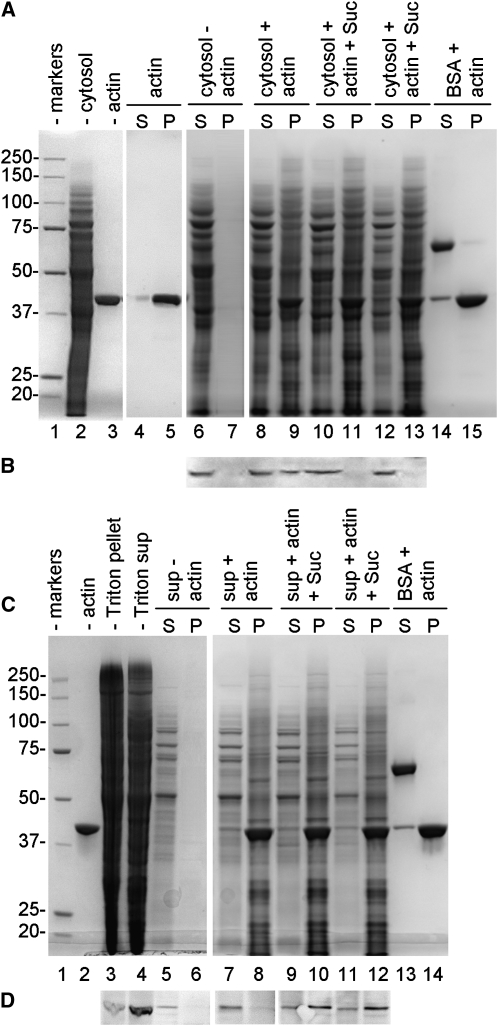
Figure 7.
Binding assay of Sus to actin filaments. A, Binding of cytoplasmic Sus. Lane 1, Molecular mass markers; lane 2, cytosolic proteins of pollen tubes (20 μg); lane 3, actin filaments (5 μg). When samples were incubated and centrifuged (S, supernatant; P, pellet), F-actin alone was essentially found in the pellet (lane 5) in comparison with the supernatant (lane 4). Conversely, cytosolic proteins alone were found in the supernatant (lane 6) but not in the pellet (lane 7). Cytosolic proteins were mixed with actin in the absence (lanes 8 and 9) or in the presence of 40 mm Suc (lanes 10 and 11) or 100 mm Suc (lanes 12 and 13). As a control, BSA was also mixed with actin filaments (lanes 14 and 15). B, Immunoblot with anti-Sus on some of the fraction shown in A. Sus was found in the unsedimented cytosolic proteins (lane 6) and in association with actin filaments in the absence of Suc (lane 9) but not in the presence of either 40 or 100 mm Suc (lanes 11 and 13). C, Binding of membrane Sus. Lane 1, Molecular mass markers. Triton-extracted proteins (lane 4; 20 μg) were mixed with actin filaments (lane 2; 5 μg) and centrifuged (S, supernatant; P, pellet). When actin filaments were omitted, membrane proteins did not sediment (lane 6). Membrane proteins were incubated with actin filaments in the absence of Suc (lanes 7 and 8) and in the presence of either 40 mm Suc (lanes 9 and 10) or 100 mm Suc (lanes 11 and 12). As a control, BSA was also mixed with actin (lanes 13 and 14). D, Immunoblot with anti-Sus on the fractions shown in C. Sus was found in the Triton-extracted protein fraction (lane 4), but it did not sediment in the absence of actin (lane 6). Sus did not sediment as well in the absence of Suc (lane 8), but it pelleted with actin in the presence of either 40 mm Suc (lane 10) or 100 mm Suc (lane 12). Blots in D are from the same nitrocellulose membrane.