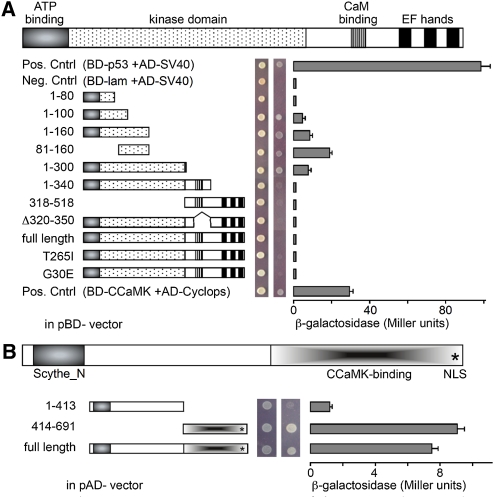
Figure 2.
CIP73 interacts with CCaMK in yeast. A, Y2H assays for interaction between CCaMK and CIP73. The kinase domain (1–300) was used in the initial isolation of CIP73 from the Y2H Library. SD-Leu-Trp medium was used for testing successful mating, and SD-Leu-Trp-His-Ade medium was used for testing the interaction. The strength of interaction was measured through the β-galactosidase activity. The combination of BD-53/AD-SV40 or BD-CCaMK/AD-CYCLOPS (Yano et al., 2008) was used as a positive control, and BD-Lam/AD-SV40 was used as a negative control (Clontech). CIP73 could only interact with CCaMK when the CaM-binding domain and three EF-hand motifs were removed from the kinase domain. The N-terminal 80 amino acid residues (80–160) of CCaMK are sufficient for interacting with CIP73 in yeast cells. B, The kinase domain of CCaMK (1–300) can interact with full-length CIP73 and CIP73-C (414–691) but cannot interact with CIP73-N (1–413). [See online article for color version of this figure.]