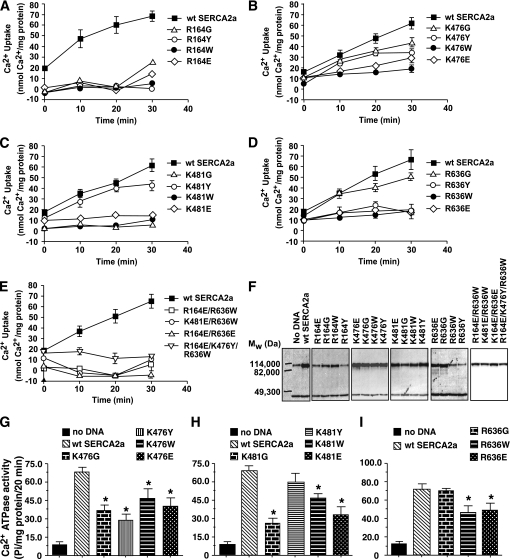
FIG. 7.
A–D: The impact of a single mutation to neutralize charge (K/R→G), simultaneously neutralizing charge and increasing hydrophilic bulk (K/R→Y), simultaneously neutralizing charge and increasing hydrophobic bulk (K/R→W), and inverting charge (K/R→E) on the ability of SERCA2a to transport Ca2+ (E1→E2). E: The impact of simultaneously neutralizing charge and increasing bulk on multiple residues on the ability of SERCA2a to transport Ca2+ (E1→E2). F: Representative autoradiograms for relevant SERCA2a mutants (upper band), emphasizing that changes in activities observed were not a result of degradation of the SERCA2a protein. The lower band represents β-actin. G–I: The impact of neutralizing charge and/or increasing bulk on amino acid residues 476, 481, and 636 on the ability of SERCA2a to hydrolyze ATP (steps 2–3 of the post-Elber’s cycle), respectively. Graphs are means ± SE from n = 4 experiments. *Significantly (P < 0.05) different from wild type.