Abstract
Background
Marijuana contains carcinogens similar to tobacco smoke and has been suggested by relatively small studies to increase the risk of head and neck cancer (HNC). Since tobacco is a major risk factor for HNC, large studies with substantial numbers of never tobacco users could help to clarify whether marijuana smoking is independently associated with HNC risk.
Methods
We pooled self-reported interview data on marijuana smoking and known HNC risk factors on 4,029 HNC cases and 5,015 controls from five case-control studies within the INHANCE Consortium. Subanalyses were conducted among never tobacco users (493 cases and 1,813 controls), and among individuals who did not consume alcohol or smoke tobacco (237 cases and 887 controls).
Results
The risk of HNC was not elevated by ever marijuana smoking (odds ratio (OR) =0.88, 95% confidence intervals (CI) =0.67, 1.16), and there was no increasing risk associated with increasing frequency, duration or cumulative consumption of marijuana smoking. An increased risk of HNC associated with marijuana use was not detected among never tobacco users (OR=0.93, 95%CI=0.63, 1.37; three studies) nor among individuals who did not drink alcohol and smoke tobacco (OR=1.06, 95%CI=0.47, 2.38; two studies).
Conclusion
Our results are consistent with the notion that infrequent marijuana smoking does not confer a risk of these malignancies. Nonetheless, because the prevalence of frequent marijuana smoking was low in most of the contributing studies, we could not rule out a moderately increased risk, particularly among subgroups without exposure to tobacco and alcohol.
Introduction
Marijuana (Cannabis sativa) is the most commonly used illegal drug in the world. It is estimated that about 160 million people consume marijuana each year, which is about 4% of the world population aged 15 to 64 (1). Since it is mainly consumed by smoking and its combustion products include tobacco carcinogens, such as nitrosamine and polycyclic aromatic hydrocarbons (benzo[α]pyrene and phenols) (2, 3) at levels that can be higher than derived from cigarettes (4), it has been suspected to be causally associated with cancers of the lung, head and neck and bladder (5). One small study observed an increased risk of upper aerodigestive tract cancers for ever marijuana smoking, with a dose response relationship for both frequency and duration of smoking (6). This association was not observed among never tobacco users and never alcohol users, but the numbers in these categories were low. The study used blood donors as controls; if these individuals tended to have less marijuana use than typical in the source population a spurious positive association would result. On the other hand, five studies, one on head and neck cancer (HNC) in New Zealand (7), one on upper aerodigestive tract cancers in Los Angeles (8), two on oral cavity cancer conducted in South England (9) and one on oral cavity cancers in the US (10) did not observe any association with ever smoking marijuana.
The INHANCE consortium was established in 2004, based on the collaboration of research groups leading large molecular epidemiology studies of HNC that were on-going or recently completed. This consortium was established to explore potential HNC risk factors that were difficult to evaluate in individual studies. The aim of this pooled analysis was to investigate the association between the risk of HNC and marijuana smoking, particularly in individuals who did not smoke tobacco or drink alcohol. Focusing on this subgroup may allow clarification on whether marijuana smoking is independently associated with HNC risk.
Methods
The INHANCE pooled data (version 1.1) included 18 individual case-control studies of HNC, of which five had information on marijuana smoking comprising 4,085 cases and 5,125 controls. The results on marijuana smoking from the Los Angeles study (601 head and neck cases and 1,040 controls) and from the Seattle study (435 cases and 615 controls), included in this pooled dataset, have already been published (8, 10). After subjects in these five studies with data missing on age, sex, or race/ethnicity, marijuana status, and cases with missing information on the site of origin of their cancer were excluded (56 cases and 110 controls), there were 4,029 cases and 5,015 controls available for the pooled analysis.
The tumor subsite distribution of cases was as follows: 981 oral cavity, 1,397 pharynx (1,165 oropharynx and 232 hypopharynx), 435 oral cavity or pharynx not otherwise specified (NOS), 1,159 larynx and 57 head and neck not otherwise specified (NOS). Two studies restricted case eligibility to squamous cell carcinomas (SCC) (Tampa and Houston studies). For other studies that provided the ICD-O-2 histological coding for each tumor (Seattle, Los Angeles and Latin America studies), we used the codes to identify SCC cases. Of the 4,029 HNC cases, 3,818 were squamous cell carcinomas (95%).
Characteristics of the individual studies included in the pooled data are presented in Table 1. Three of the five studies were hospital-based case-control studies. Four of the studies frequency-matched controls to cases based on age and sex. The Latin America study additionally matched on study center. The Los Angeles study individually matched controls to cases based on age decade, sex, and neighborhood. All interviews were conducted face-to-face with structured questionnaires. Questionnaires were collected from all individual studies, to assess the comparability of the data and wording of interview questions. Anonymized data from individual studies were pooled; each data item was checked for illogical or missing values; inconsistencies were resolved.
Table 1.
Summary of individual studies in INHANCE pooled data v1.1
| Study Location |
Recruitment period |
Cases |
Controls1 |
|||
|---|---|---|---|---|---|---|
| Source | Participation rate |
Age eligibility |
Source | Participation rate |
||
| Seattle, WA | 1985–1995 | Cancer registry |
54%, 63%2 | 18–65 | Random digit dialing |
63%, 61%2 |
| Tampa, FL | 1999–2003 | Hospital | 98% | ≥18 | Cancer screening clinic - healthy |
90% |
| Los Angeles, CA |
1999–2004 | Cancer registry |
49% | 18–65 | Neighborhood | 68% |
| Houston, TX | 2001–2006 | Hospital | 95% | ≥18 | Hospital visitors |
80% |
| Havana, Buenos Aires, Brazil |
2000–2003 | Hospital | 95% | 15–79 | Hospital - patients |
86% |
All studies frequency matched controls to cases, minimally on age and sex. Additional frequency matching factors included center (Latin America), ethnicity and neighborhood (Los Angeles study).
Two response rates are reported because data were collected in two population-based case – control studies, the first from 1985 to 1989 among men and the second from 1990 to 1995 among men and women.
Data on whether an individual had smoked marijuana smoking, and at what frequency and length of time, were collected differently across studies. The questions asked for assessing marijuana smoking were: “Have you ever used marijuana?” (Los Angeles, Seattle and Houston studies); “Have you ever smoked marijuana at least once per week for 6 months?” (Latin America study); and “Have you ever smoked marijuana at least once a day for one years time?” (Tampa study).
The Houston and Tampa studies asked each subject to report the frequency and years of marijuana use average over his/her lifetime, while three studies (Seattle, Latin America, and Los Angeles) obtained information about different periods of marijuana smoking over the subject’s lifetime; for these three studies, the lifetime average was calculated by weighting the frequency of the specific period by the duration of that period and total years of marijuana smoking were calculated by summing across the durations of the individual periods. A “joint-year” variable was created and defined as the number of joints per day multiplied by the duration of marijuana smoking in years.
Statistical analysis
The association between marijuana smoking and the risk of HNC was assessed by computing odds ratios (OR) and 95% confidence intervals (CI) from unconditional logistic regression models for each case-control study. To adjust for potential confounders, the models included age (categorical), sex, education (categorical), race/ethnicity, study center, pack-years (continuous), duration of smoking pipe (continuous), duration of smoking cigar (continuous) and duration of alcohol drinking in years (continuous).
Stratified analyses were conducted by subsite of HNC (oral cavity, pharynx, oral cavity/pharynx NOS and larynx). Additional analyses were restricted to never tobacco users (493 cases and 1,813 controls), never alcohol drinkers (568 cases and 1,505 controls) and never tobacco and never alcohol drinkers (237 cases and 887 controls) based on the definitions described previously (11).
For subjects missing data on education level (305 cases and 212 controls), we applied multiple imputations (five imputations) with the PROC MI procedure in SAS. We used the logistic regression model (12) to predict education level with age, sex, race/ethnicity, study, and case/control status for the Latin American and North American regions separately. The logistic regression results to assess summary estimates for marijuana smoking for the five imputations were combined by using the PROC MIANALYZE procedure.
We tested for heterogeneity between studies for each analysis, using a log likelihood ratio test. We compared the model with and without a product term between marijuana smoking and the study indicator. We then compared twice the difference between the log likelihood of these two models to a chi-squared distribution with degree of freedom equal to the number of studies minus one. When the heterogeneity p was below 0.05, study-specific estimates were included in a two-stage random-effects logistic regression model. Influence analyses were conducted, with exclusion of each study one at a time, to evaluate if the magnitude of the estimate was dependent on any one study.
RESULTS
Approximately 10% of cases and 15% of controls were ever marijuana smokers (Table 2). The greatest proportion of ever marijuana smokers was observed in the Los Angeles study (58.7% of the cases and 54.2% of the controls). There were higher proportions of marijuana smokers among white men, subjects 45–55 years old, and subjects with an education level greater than college. Among never tobacco users, 13.9% of cases and 29.7% of controls reported ever smoking marijuana. Among never alcohol drinkers, 8.4% of cases and 10.8% of controls reported ever smoking marijuana.
Table 2.
Characteristics of head and neck cancer cases and controls
| Cases |
Controls | |||||
|---|---|---|---|---|---|---|
| Total | No ever marijuana smokers 1 |
% ever marijuana smokers |
Total | No ever marijuana smokers1 |
% ever marijuana smokers |
|
| Total | 4029 | 408 | 10.1 | 5015 | 744 | 14.8 |
| Study | ||||||
| Seattle | 407 | 68 | 16.7 | 607 | 103 | 17.0 |
| Tampa | 207 | 5 | 2.4 | 897 | 4 | 0.4 |
| Los Angeles | 416 | 244 | 58.7 | 1002 | 543 | 54.2 |
| Houston | 829 | 49 | 5.9 | 865 | 64 | 7.4 |
| Latin America | ||||||
| Buenos Aires | 334 | 0 | 0.0 | 188 | 5 | 2.7 |
| Havana | 196 | 1 | 0.5 | 176 | 2 | 1.1 |
| Goiania | 391 | 7 | 1.8 | 242 | 4 | 1.7 |
| Pelotas | 128 | 1 | 0.8 | 225 | 0 | 0.0 |
| Porto Alegre | 191 | 2 | 1.0 | 152 | 1 | 0.7 |
| Rio de Janeiro | 428 | 12 | 2.8 | 241 | 7 | 2.9 |
| Sao Paulo | 502 | 19 | 3.8 | 420 | 11 | 2.6 |
| Age Categories | ||||||
| <40 | 162 | 39 | 24.1 | 347 | 90 | 25.9 |
| 40–<45 | 281 | 53 | 18.9 | 455 | 131 | 28.8 |
| 45–<50 | 526 | 115 | 21.9 | 643 | 156 | 24.3 |
| 50–<55 | 690 | 95 | 13.8 | 945 | 208 | 22.0 |
| 55–<60 | 805 | 92 | 11.4 | 992 | 136 | 13.7 |
| >=60 | 1565 | 14 | 0.9 | 1633 | 23 | 1.4 |
| Sex | ||||||
| Women | 785 | 66 | 8.4 | 1547 | 213 | 13.8 |
| Men | 3244 | 342 | 10.5 | 3468 | 531 | 15.3 |
| Race | ||||||
| White non Hispanic | 1526 | 280 | 18.3 | 2684 | 558 | 20.8 |
| Black | 145 | 52 | 35.9 | 271 | 71 | 26.2 |
| Hispanic | 141 | 27 | 19.1 | 328 | 68 | 20.7 |
| Asian + other | 47 | 10 | 21.3 | 88 | 17 | 19.3 |
| Latin American2 | 2170 | 42 | 1.9 | 1644 | 30 | 1.8 |
| Education | ||||||
| < Junior high school | 1722 | 46 | 2.7 | 1310 | 29 | 2.2 |
| Some high school | 441 | 66 | 15.0 | 390 | 49 | 12.6 |
| High School Graduate | 475 | 77 | 16.2 | 666 | 128 | 19.2 |
| Vocational, some college | 606 | 126 | 20.8 | 1159 | 242 | 20.9 |
| ≥college | 480 | 90 | 18.8 | 1278 | 296 | 23.2 |
| Missing | 305 | 3 | 1.0 | 212 | / | / |
|
Tobacco smoking status |
||||||
| Never | 493 | 57 | 11.6 | 1813 | 221 | 12.2 |
| Ever | 3536 | 351 | 9.9 | 3196 | 522 | 16.3 |
| Missing | / | / | 6 | 1 | ||
|
Alcohol Drinking status |
||||||
| Never | 568 | 34 | 6.0 | 1505 | 80 | 5.3 |
| Ever | 3457 | 373 | 10.8 | 3506 | 663 | 18.9 |
| Missing | 4 | 1 | 4 | 1 | ||
Ever marijuana smoking was defined differently in the studies: the Seattle, Houston and Los Angeles studies (‘ever’ use), Latin America study (≥once/week for 6 months) and Tampa study (≥once/day for 1 year).
The Latin America study did not assess race/ethnicity, thus we classified the subjects as a separate category “Latin Americans”.
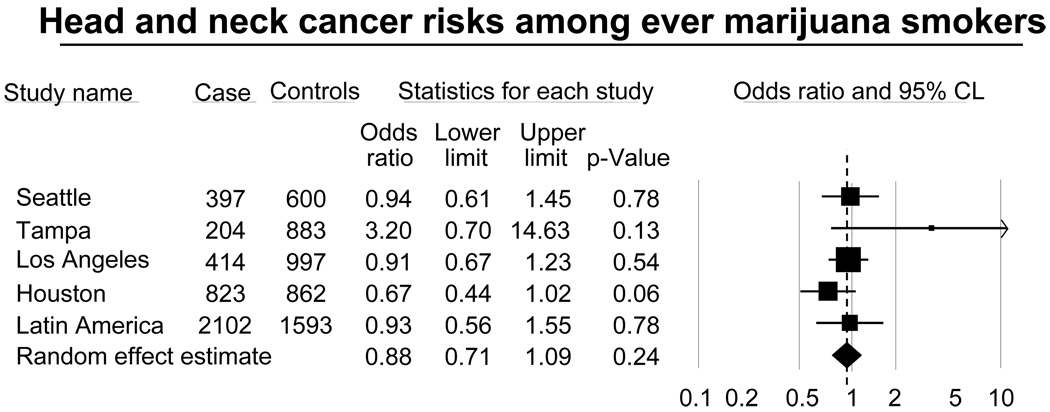
We did not observe an association with ever marijuana smoking and the risk of HNC (OR=0.88, 95%CI= 0.67, 1.16; Table 3). Figure 1 shows a forest plot of the study specific estimates of the risk of HNC associated with marijuana smoking. All five studies failed to detect an association between HNC and marijuana smoking. Only the Tampa study had an OR above three while the other studies showed OR below one.
Tables 3.
Marijuana smoking and the risk of head and neck cancer
| Case | Controls | OR* | 95% CI | |
|---|---|---|---|---|
| Total | 4029 | 5015 | ||
| Marijuana smoking | ||||
| Never | 3538 | 4199 | 1.00 | Ref |
| Ever | 402 | 736 | 0.88 | (0.67, 1.16) |
| Missing | 89 | 80 | ||
| p for heterogeneity | 0.07 | |||
| Frequency of marijuana smoking (times per day)~ | ||||
| Never | 3339 | 3319 | 1.00 | |
| 0–1 | 298 | 630 | 0.87 | (0.61, 1.25) |
| >1–3 | 49 | 61 | 0.71 | (0.35, 1.47) |
| >3 | 42 | 42 | 0.87 | (0.40, 1.89) |
| Missing | 94 | 66 | ||
| p for trend | 0.26 | |||
| p for heterogeneity | 0.03 | |||
| Duration of marijuana smoking (in years)~ | ||||
| Never | 3339 | 3319 | 1.00 | Ref |
| >0–5 | 150 | 319 | 0.81 | (0.53, 1.23) |
| >5–10 | 65 | 129 | 0.87 | (0.48, 1.57) |
| >10–20 | 74 | 145 | 0.82 | (0.46, 1.44) |
| >20 | 100 | 140 | 0.94 | (0.53, 1.66) |
| Missing | 94 | 66 | ||
| p for trend | 0.77 | |||
| p for heterogeneity | 0.36 | |||
| Cumulative exposure (joint-year) #~ | ||||
| Never | 3339 | 3319 | 1.00 | Ref |
| >0–2 | 208 | 476 | 0.89 | (0.60, 1.31) |
| >2–5 | 36 | 77 | 0.70 | (0.31, 1.56) |
| >5 | 145 | 180 | 0.86 | (0.54, 1.37) |
| Missing | 94 | 66 | ||
| p for trend | 0.22 | |||
| p for heterogeneity | 0.04 | |||
Random effect estimates. Adjusted for age (categorical), sex, race, education level, study, packyear (continuous), alcohol duration (continuous), duration of smoking pipe (continuous), duration of smoking cigar (continuous). Likelihood Heterogeneity test by study.
Tampa study excluded
A joint-year is the number of joints per day multiplied by the duration of marijuana smoking in years (1 joint-year being equivalent to 1 joint per day for one year or 365 joints lifetime).
Figure 1.
The risk of head and neck cancer associated with ever marijuana smoking by study.
Odds ratios were adjusted on age, sex, race/ethnicity, education level, study, packyears of tobacco smoking, years of alcohol drinking, years of cigar smoking and years of pipe smoking.
Squares = study-specific odds ratios;
Size of the square = the weight given to this study (inverse of the variance of the log odds ratio) when estimating the summary odds ratio;
Horizontal lines = study-specific confidence intervals (CIs);
Diamond = summary estimate combining the study-specific estimates with a random-effects model;
Solid vertical line = odds ratio of 0.1, 0.5, 1, 2 and 10;
Dashed vertical line = summary odds ratio.
When we restricted the analysis to studies with similar definitions of “ever use” (Los Angeles, Seattle and Houston studies), we did not observe an association between ever marijuana smoking and HNC risk (OR=0.85, 95%CI= 0.53, 1.35). When we applied a specific cut-off definition of marijuana smoking to 1 joint per day for 1 year, we similarly did not observe an association for ever marijuana smoking. In addition, we did not observe any dose-response trend for frequency of marijuana smoking, marijuana smoking duration or cumulative marijuana consumption in these analyses. The Tampa study was excluded from the analysis on duration and frequency of marijuana smoking since there were not enough cases or controls in each category of frequency and duration of marijuana smoking to calculate these estimates. Heterogeneity was detected between studies for the associations of frequency of marijuana smoking, and joint-years of marijuana smoking with risk of head and neck cancers.
Increased risks were not observed by ever, frequency, duration or cumulative marijuana smoking, for any HNC subsite (Table 4). For pharyngeal cancers, the Tampa and Seattle studies were excluded from the analysis on frequency of marijuana use and the Seattle study was excluded for the analysis on duration and cumulative consumption since there were not enough cases or controls in the categories of frequency and duration of marijuana smoking. For oropharyngeal cancer, we observed an increased risk associated with ever marijuana smoking (OR=1.40, 95%CI= 1.05, 1.87), but a dose-response relationship was not detected.
Table 4.
Marijuana smoking and the risk of head and neck cancer stratified by subsite of cancer
| Oral cavity | Pharynx#* | Larynx~ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR | 95% CI | Cases | Controls | OR | 95% CI | Cases | Controls | OR | 95% CI | |
| Total | 981 | 5015 | 1397 | 5015 | 1159 | 4408 | ||||||
| Marijuana smoking | ||||||||||||
| Never | 877 | 4199 | 1.00 | Ref | 1177 | 4199 | 1.00 | Ref | 1063 | 3701 | 1.00 | Ref |
| Ever | 77 | 736 | 0.74 | (0.55, 1.00) | 192 | 736 | 1.13 | ( 0.76, 1.68) | 72 | 634 | 0.98 | (0.69, 1.39) |
| Missing | 27 | 80 | 28 | 80 | 24 | 73 | ||||||
| p for heterogeneity | 0.33 | 0.03 | 0.62 | |||||||||
| Frequency of marijuana smoking (times per day) | ||||||||||||
| Never | 877 | 4199 | 1.00 | Ref | 988 | 2821 | 1.00 | Ref | 1063 | 3701 | 1.00 | Ref |
| 0–1 | 59 | 631 | 0.76 | (0.54, 1.07) | 113 | 534 | 1.05 | (0.49, 2.22) | 47 | 535 | 0.88 | (0.57, 1.34) |
| >1–3 | 10 | 61 | 0.63 | (0.30, 1.30) | 22 | 56 | 1.01 | (0.28, 3.64) | 11 | 56 | 1.08 | (0.54, 2.18) |
| >3 | 7 | 43 | 0.64 | (0.27, 1.52) | 14 | 41 | 0.82 | (0.19, 3.61) | 12 | 42 | 1.05 | (0.51, 2.15) |
| Missing | 28 | 81 | 24 | 59 | 26 | 74 | ||||||
| p for trend | 0.04 | 0.71 | 0.99 | |||||||||
| p for heterogeneity | 0.85 | <0.01 | 0.55 | |||||||||
| Duration of marijuana smoking (in years) | ||||||||||||
| Never | 877 | 4199 | 1.00 | Ref | 1124 | 3319 | 1.00 | Ref | 1063 | 3701 | 1.00 | Ref |
| >0–5 | 29 | 319 | 0.64 | (0.42, 0.99) | 75 | 319 | 1.08 | (0.63, 1.85) | 20 | 278 | 0.65 | (0.38, 1.11) |
| >5–10 | 16 | 129 | 0.83 | (0.46, 1.50) | 28 | 129 | 1.01 | (0.46, 2.24) | 12 | 111 | 1.10 | (0.56, 2.14) |
| >10–20 | 18 | 145 | 0.80 | (0.46, 1.40) | 36 | 145 | 1.07 | (0.51, 2.26) | 10 | 111 | 0.85 | (0.41, 1.76) |
| >20 | 13 | 143 | 0.74 | (0.39, 1.39) | 45 | 140 | 1.31 | (0.62, 2.76) | 28 | 134 | 1.42 | (0.84, 2.38) |
| Missing | 28 | 80 | 26 | 66 | 26 | 73 | ||||||
| p for trend | 0.15 | 0.66 | 0.32 | |||||||||
| p for heterogeneity | 0.93 | 0.23 | 0.69 | |||||||||
| Cumulative exposure (joint-year) | ||||||||||||
| Never | 877 | 4199 | 1.00 | Ref | 1124 | 3319 | 1.00 | Ref | 1063 | 3701 | 1.00 | Ref |
| >0–2 | 41 | 476 | 0.73 | (0.50, 1.08) | 97 | 476 | 1.15 | (0.68, 1.94) | 31 | 406 | 0.84 | (0.52, 1.36) |
| >2–5 | 9 | 77 | 0.68 | (0.32, 1.46) | 21 | 77 | 1.29 | (0.45, 3.75) | 3 | 66 | 0.31 | (0.09, 1.07) |
| >5 | 26 | 182 | 0.73 | (0.46, 1.16) | 66 | 180 | 1.03 | (0.47, 2.26) | 36 | 161 | 1.20 | (0.77, 1.85) |
| Missing | 28 | 81 | 26 | 66 | 26 | 74 | ||||||
| p for trend | 0.07 | 0.76 | 0.75 | |||||||||
| p for heterogeneity | 0.46 | 0.08 | 0.21 | |||||||||
Adjusted for age (categorical) study, race, sex, education level, packyear (continuous), alcohol duration (continuous), duration of smoking pipe (continuous) and duration of smoking cigar (continuous)
Random effect estimates
The Seattle study was not included in the analysis for duration and cumulative consumption of marijuana. The Tampa and Seattle studies were not included in the analysis on frequency of marijuana consumption.
No Laryngeal cases in Seattle (the number of controls for laryngeal cancer is different because the Seattle study is not included in the laryngeal cancer analysis)
In the analysis restricted to never tobacco users (353 cases and 1017 controls; Table 5), the Tampa study was not included because all marijuana smokers were also tobacco users. The Latin America study was also excluded because no cases and only one control smoked marijuana without using tobacco. No association between smoking marijuana and the risk of HNC was observed among never tobacco users. Among never alcohol drinkers, an increased risk was observed for subjects who smoked marijuana for more than 20 years (trend p =0.05) and for subjects who smoked more than 5 joint-years of marijuana (trend p=0.07). Dose-response relationships for frequency, duration or cumulative consumption of marijuana use were not observed with head and neck cancer risk among never tobacco users.
Table 5.
Marijuana smoking and the risk of head and neck cancer by tobacco and alcohol status
| Never tobacco users1* | Never alcohol users2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR | 95% CI | Cases | Controls | OR | 95% CI | |
| Total | 353 | 1017 | 345 | 997 | ||||
| Marijuana smoking | ||||||||
| Never | 295 | 797 | 1.00 | Ref | 311 | 915 | 1.00 | Ref |
| Ever | 57 | 220 | 0.93 | (0.63, 1.37) | 34 | 79 | 1.27 | (0.77, 2.12) |
| Missing | 1 | 0 | 0 | 3 | ||||
| p for heterogeneity | 0.21 | 0.06 | ||||||
| Frequency of marijuana smoking (times per day) | ||||||||
| Never | 295 | 797 | 1.00 | Ref | 311 | 915 | 1.00 | Ref |
| 0–1 | 52 | 205 | 0.93 | (0.62, 1.39) | 26 | 68 | 1.30 | (0.74, 2.28) |
| >1 | 5 | 15 | 0.92 | (0.31, 2.68) | 8 | 11 | 1.18 | (0.43, 3.19) |
| Missing | 1 | 0 | 0 | 3 | ||||
| p for trend | 0.72 | 0.42 | ||||||
| p for heterogeneity | 0.06 | 0.22 | ||||||
| Duration of marijuana smoking (in years) | ||||||||
| Never | 295 | 797 | 1.00 | Ref | 311 | 915 | 1.00 | Ref |
| >0–5 | 31 | 113 | 0.99 | (0.62, 1.57) | 12 | 45 | 0.86 | (0.42, 1.77) |
| >5–10 | 9 | 47 | 0.69 | (0.32, 1.51) | 6 | 11 | 1.22 | (0.41, 3.61) |
| >10–20 | 6 | 35 | 0.60 | (0.24, 1.50) | 6 | 13 | 1.38 | (0.47, 4.04) |
| >20 | 11 | 25 | 1.60 | (0.73, 3.52) | 10 | 10 | 3.12 | (1.17, 8.36) |
| Missing | 1 | 0 | 0 | 3 | ||||
| p for trend | 0.99 | 0.05 | ||||||
| p for heterogeneity | 0.53 | 0.18 | ||||||
| Cumulative exposure (joint-year) | ||||||||
| Never | 295 | 797 | 1.00 | Ref | 311 | 915 | 1.00 | Ref |
| >0–2 | 41 | 176 | 0.87 | (0.57, 1.33) | 16 | 59 | 0.99 | (0.52, 1.88) |
| >2–5 | 5 | 18 | 0.89 | (0.31, 2.53) | 3 | 10 | 0.58 | (0.15, 2.26) |
| >5 | 11 | 26 | 1.34 | (0.62, 2.92) | 15 | 10 | 3.25 | (1.31, 8.02) |
| Missing | 1 | 0 | 0 | 3 | ||||
| p for trend | 0.80 | 0.07 | ||||||
| p for heterogeneity | 0.17 | 0.23 | ||||||
Never tobacco user are never user of cigarette, pipe, cigar, chew and snuff. Does not include Tampa and Latin America studies
OR adjusted for age (categorical), sex, race, study, education_level, alcohol_duration (continuous). Does not include Tampa and Latin America studies
OR adjusted for age (categorical), sex, race, study, education_level, packyear (continuous) duration of smoking pipe (continuous) and duration of smoking cigar (continuous). Does not include Seattle and Latin America studies
In the analysis restricted to never alcohol users (345 cases and 997 controls; Table 5), the Seattle study was not included because all marijuana smokers were also alcohol drinkers. The Latin America study was also not included since no cases and only one control smoked marijuana without drinking alcohol. We observed almost no association between ever marijuana smoking and HNC risk (OR=1.33, 95%CI=0.77, 2.12). However, we observed an increased risk of HNC associated with smoking marijuana for more than 20 years (OR=3.12, 95%CI=1.17–8.36), with a dose-response trend suggested (p=0.05) and an increased risk associated with cumulative consumption of more than 5 joint-years (OR=3.26, 95%CI=1.32, 8.06).
In the analysis restricted to never alcohol and never tobacco users, only the Houston and the Los Angeles studies had information on marijuana smoking for both cases and controls (149 cases and 407 controls). The OR for ever marijuana smoking was 1.06 (95%CI=0.47, 2.38). Association with frequency and duration of marijuana smoking could not be assessed in this group due to the limited numbers of subjects (only 10 cases and 33 controls used marijuana and were never tobacco users and never alcohol drinkers).
Stratification by sex, region (North America, Latin America), age (<50, ≥50), control type (hospital-based or population-based) or study period (before 2000 or after 2000) did not result in differences in the OR for ever marijuana smoking across the different strata.
For the ORs for ever marijuana use, additional adjustment for ever tobacco chewing (OR=0.88, 95%CI= 0.62, 1.23; Tampa, Houston, Los Angeles and Seattle studies), ever use of snuff (OR=0.85, 95%CI=0.53, 1.14; Los Angeles, Houston and Seattle studies), passive smoking exposure (OR=0.89, 95%CI=0.49, 1.61; Houston, Los Angeles and Latin America studies), BMI (OR=0.84, 95%CI=0.59, 1.21; Houston, Los Angeles, Tampa and Latin America studies) and family history of HNC (OR=0.85, 95%CI=0.59, 1.21; Houston, Los Angeles, Tampa and Latin America studies) did not change the results. The results for the frequency and duration of marijuana smoking also remained unchanged with these adjustments.
DISCUSSION
In our pooled analysis, we did not observe an association between marijuana smoking and the risk of HNC. Similarly, we did not observe an association among never tobacco users. Among never alcohol users, we observed an increased risk of HNC for smoking marijuana for more than 20 years; although we adjusted for tobacco use, we cannot rule out the possibility of residual confounding by tobacco.
We also did not observe an association between HNC risk and marijuana smoking when restricting the analysis to never tobacco and never alcohol users, but these results were based on only two studies, with low statistical precision. Tobacco smoking and alcohol drinking were associated with marijuana use among controls and cases. The controls in our study who were ever tobacco smokers had a higher proportion of ever marijuana use (16%) compared to the controls who were never tobacco smokers (12%). Controls who were ever drinkers had a higher prevalence of ever marijuana use (19%) compared to controls who were never drinkers (5%). However, the mean packyears of tobacco smoking and frequency of alcohol drinking (in drinks per day) was greater among the never-marijuana users than ever-marijuana users among controls. The associations are further complicated by the strong combined effect of tobacco and alcohol on the risk of head and neck cancer.
Alhough the direction of the bias in our estimates is difficult to predict, bias due to measurement error must be present and bias due to differential selection or residual confounding cannot be ruled out. Human papillomavirus has been suggested to be a risk factor for HNC and more specifically for oropharyngeal cancer (13). We were not able to account for this risk factor, but we observed an association between oropharyngeal cancer and marijuana smoking that was not confirmed by a dose response relation. Recently, Gillison et al (14) showed a strong association between marijuana smoking and HPV-16 positive squamous cell carcinoma of the head and neck (HNSCC). They suggested that cannabinoids might promote the development of a HPV-16 positive HNSCC through decreased immune function. Though other exposures such as sexual history, tobacco and alcohol were adjusted, the association could reflect residual confounding by these exposures. These alternative hypotheses could be explored by collecting data on both HPV and potential confounders of the association.
Although pooling data across several studies provided a larger number of HNC cases and controls than previous studies, there were several limitations inherent in pooled analyses. Our major concern was the heterogeneity across studies, especially due to differences in the definition of ever marijuana smoking, and differences in social acceptance of marijuana smoking. Differences in the social acceptance of smoking marijuana may lead to differential misclassification across countries or regions. Marijuana is illegal in all of the countries included in this analysis, but to very different degrees, and it is not clear whether cases and controls may differ in the way they report their marijuana consumption. Heterogeneity was detected for the associations of frequency and joint-years of marijuana smoking with the risk of head and neck cancer.
From the definition of marijuana smoking, three of the studies could detect individuals who smoked even one joint in a lifetime, while the two other studies used definitions that attempted to capture regular marijuana smoking (once per week over six months or once per day over one year). The inclusion of moderate marijuana smokers in the reference category might have diluted the true association. For a common definition for ever marijuana smoking, we applied the highest cut-off (once per day for one year) across studies and did not observe an association between ever smoking and the risk of HNC.
The pattern of marijuana smoking is different compared to other smoking products. While tobacco use is clearly addictive, with a high frequency and level of exposure needed to avoid withdrawal symptoms (15), marijuana smoking is often recreational, with the purpose of attaining an effect of euphoria which is reached with low levels of frequency and duration (16). Thus, despite the large size of our population, we lacked sufficient numbers of individuals who had smoked more than 5 joints per day and for more than 20 years, limiting our ability to assess the risk of HNC among heavy marijuana smokers.
Another possible reason that we did not observe an association between HNC risk and marijuana smoking is that aside from the Los Angeles study, there was a low proportion of marijuana smokers. The individual studies did not have enough statistical precision to detect or exclude an odds ratio of 1.2 for HNC risk. Our estimates for HNC risk do not exclude the possibility of a modest OR for ever marijuana smokers. We also did not have adequate data to distinguish possible differences in effect due to different forms of smoking (joints, pipes, water pipes). Furthermore, we had no data on variation in the weight or potency of “joints” across countries.
Marijuana smoke contains carcinogens similar to those in cigarette smoke (3, 17, 18). Some studies suggested that the tar contained in the smoke of marijuana is higher than that of cigarette (4, 17). On the other hand, Hall et al. reported that there is little mechanistic evidence that Δ9− tetrahydrocannabinol (THC), the main psychoactive molecule of cannabis, or other cannabinoids have mutagenic or carcinogenic effects (19). Several studies have even suggested an anticarcinogenic effect of cannabis. According to Blazquez et al. (20), cannabinoids might inhibit VEGF pathway, by reducing the expression 10 genes related directly or indirectly to the VEGF pathway in mouse gliomas, and thus reduce angiogenesis. Melamede et al. suggested that cannabinoids might also down-regulate immunologically-generated free radical production (21). Thus, the carcinogenic effect of tar could be reduced by the anticancer mechanisms involving Δ9-THC. Additionally, if cannabinoids promote the development of a HPV-16 positive HNSCC , as reported by Gillison et al., perhaps the association is relevant only in certain subgroups of HNC patients. It may be possible that the suppression of some aspects of immune function leads to a weaker response to the HPV infection, which leads to increased HNC risk. These mechanisms might explain the absence of an association between marijuana smoking and HNC risk overall.
In conclusion, we did not find evidence of a positive association between marijuana smoking and the risk of HNC. In an attempt to exclude the possibility of residual confounding from major risk factors, we restricted our analysis to never tobacco users and never alcohol users, but still did not detect associations. Nonetheless, because the prevalence of frequent marijuana smoking was low in most of the contributing studies, we lacked precision to rule out a moderately increased risk, particularly among subgroups lacking exposure to tobacco and alcohol.
Acknowledgements
This work was supported by a grant from the US National Institutes of Health, National Cancer Institute (R03CA113157). Dr. Yuan-chin Amy Lee was supported by a Special Training Award from the International Agency for Research on Cancer.
The individual studies were funded by the following grants:
Seattle study: US NIH grants R01CA048896 and R01DE012609
Tampa study: US NIH grants P01CA068384 and K07CA104231
Los Angeles study: US NIH grants P50CA90388, R01DA11386, R03CA77954, T32CA09142, U01CA96134, and R21ES011667, as well as the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center
Houston study: US NIH grants R01ES11740 and R01CA100264
Latin America study: FONCYT (Fondo para la Investigacion Cientifica y Tecnologica) Argentina, IMIM (Barcelona), Fundação de Amparo à Pesquisa no Estado de São Paulo (FAPESP) No 01/01768-2, European Commission (IC18-CT97-0222)
Reference List
- 1.United Nations Office of Drug and Crime. United Nations, editor. Cannabis Market - Abuse. Geneva: United Nations Publication; World Drug Report. 2007:114–121.
- 2.IARC working group on the Evaluation of Carcinogenic Risks to Humans. IARC press, editor. Lyon: World Health Organization; Tobacco smoke and involuntary smoking. 2004 [PMC free article] [PubMed]
- 3.Hoffmann D, Brunnemann D, Gori G, Wynder E. On the carcinogenecity of marijuana smoke. Recent Adv Phytochem. 1975;9:63–81. [Google Scholar]
- 4.Rickert WS, Robinson JC, Rogers B. A comparison of tar, carbon monoxide and pH levels in smoke from marihuana and tobacco cigarettes. Can J Public Health. 1982;73:386–391. [PubMed] [Google Scholar]
- 5.Chacko JA, Heiner JG, Siu W, Macy M, Terris MK. Association between marijuana use and transitional cell carcinoma. Urology. 2006;67:100–104. doi: 10.1016/j.urology.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZF, Morgenstern H, Spitz MR, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8:1071–1078. [PubMed] [Google Scholar]
- 7.Aldington S, Harwood M, Cox B, et al. Cannabis use and cancer of the head and neck: Case-control study. Otolaryngol Head Neck Surg. 2008;138:374–380. doi: 10.1016/j.otohns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashibe M, Morgenstern H, Cui Y, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–1834. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- 9.Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for oral cancer in newly diagnosed patients aged 45 years and younger: a case-control study in Southern England. J Oral Pathol Med. 2004;33:525–532. doi: 10.1111/j.1600-0714.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64:4049–4054. doi: 10.1158/0008-5472.CAN-03-3425. [DOI] [PubMed] [Google Scholar]
- 11.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 12.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John WIley and Sons,Inc.; 1987. [Google Scholar]
- 13.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 14.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Substance use disorders. In: American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Publication; pp. 191–297. [Google Scholar]
- 16.Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 17.Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347–351. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]
- 18.Tashkin DP, Gliederer F, Rose J, et al. Tar, CO and delta 9THC delivery from the 1st and 2nd halves of a marijuana cigarette. Pharmacol Biochem Behav. 1991;40:657–661. doi: 10.1016/0091-3057(91)90378-f. [DOI] [PubMed] [Google Scholar]
- 19.Hall W, MacPhee D. Cannabis use and cancer. Addiction. 2002;97:243–247. doi: 10.1046/j.1360-0443.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 20.Blazquez C, Gonzalez-Feria L, Alvarez L, et al. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–5623. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- 21.Melamede R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct J. 2005;2:21. doi: 10.1186/1477-7517-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]