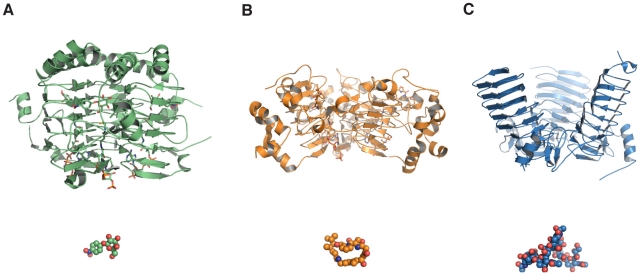
Figure 5. Inclination angles of LβH-acetyltransferases correlate with the size of the acceptor substrate.
(A) The galactoside acetyltransferase GAT (PDB-ID: 1KRV) [23]. (B) The streptogramin acetyltransferase Vat(D) (PDB-ID: 1KHR) [24]. (C) The polysialic acid acetyltransferase NeuO (PDB-ID: 3JQY). The different structures are displayed in cartoon mode with their individual substrate displayed as spheres in the same color. Whereas the LβH-fold is highly conserved also with respect to the acetyl-CoA binding site, the inclination angle between the subunits increases with the size of the acceptor substrate and is by far most pronounced in NeuO.