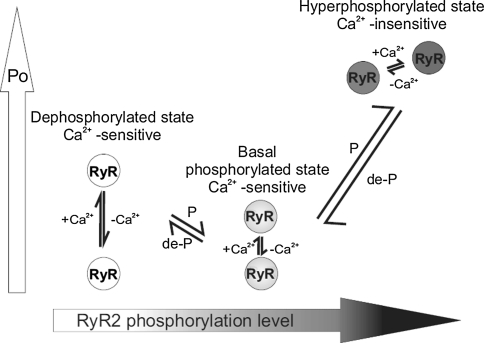
Fig. 8.
Model summarizing the relationship between the phosphorylation level of RyR2 and channel gating behavior. Large vertical arrow on the left indicates increasing P o, and large horizontal arrow indicates increasing RyR2 phosphorylation levels. At the basal levels of phosphorylation, channel P o is maintained at a low level and the channel gates in a Ca2+-sensitive manner, fully responsive to the levels of other channel regulators. Lowering the free [Ca2+] to subactivating levels (<1 nM) reduces P o to zero. The fully dephosphorylated channel (dephosphorylated state) gates with a higher P o than the channel phosphorylated at basal levels but remains sensitive to cytosolic Ca2+ (again, lowering the free [Ca2+] to subactivating levels [<1 nM] reduces P o to zero). When the channel is maximally phosphorylated (hyperphosphorylated state), exceptionally long open states result, leading to high P o values. In this conformation, RyR2 is uncoupled from the usual regulatory effects of cytosolic Ca2+ and cannot be closed by lowering cytosolic [Ca2+] below activating levels. P and de-P indicate phosphorylation and dephosphorylation, respectively