Figure 1.
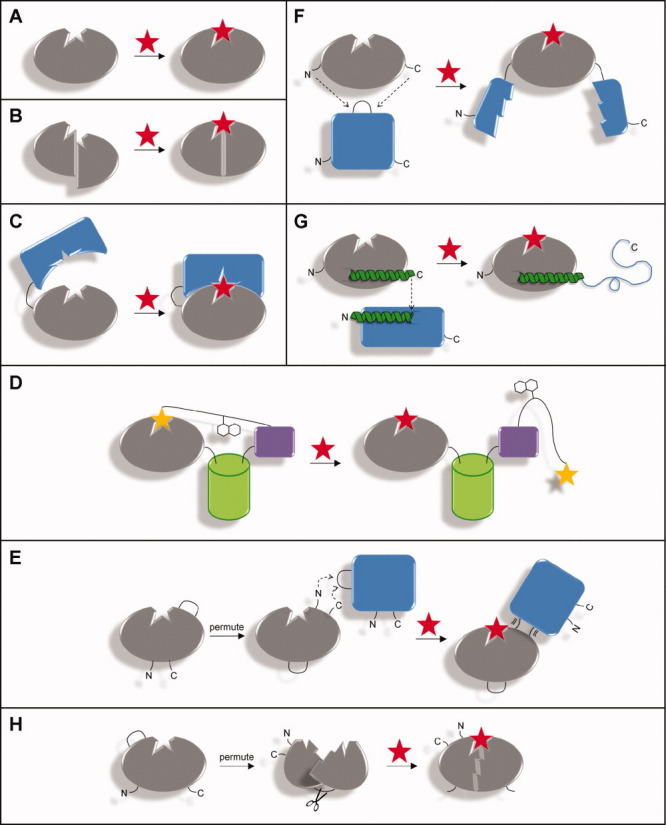
Approaches for converting a binding protein into a switch. The binding protein is shown in gray and the ligand as a red star. (A) Lock-and-key binding of an unmodified protein, in which the free and bound conformations are identical. (B) Altering specificity of a protein with a pre-existing conformational change. (C) Affinity clamp technology. An enhancer domain (blue) is fused to the binding protein via a short linker (black line). The open and closed forms are shown at left and right, respectively. (D) SNAP-tag methodology. The binding protein is joined to an FP (green) and SNAP (purple). A synthetic molecule, consisting of a chemical analog of the ligand (yellow star), a fluorescent acceptor, and O6-benzylguanine (covalently bound to SNAP) links the binding protein to SNAP. Displacement of the ligand analog causes the protein to switch from the closed (left) to the open (right) form. (E) Protein-in-protein insertion with circular permutation. In this example, the binding protein is first permuted so that its new termini are positioned close to the binding site. It is then inserted into a surface loop of the reporter protein (blue), to which binding-induced conformational changes are propagated through the nascent linkage. (F) Mutually exclusive folding by domain insertion. Ligand binding causes the binding protein to fold, which mechanically unfolds the reporter protein (blue). (G) Overlapping sequences. The C-terminus of the binding protein and the N-terminus of the reporter protein (blue) contain an α-helix of similar amino acid sequence. The C-terminus of the binding protein is linked to the N-terminus of the reporter protein such that they share a portion of that α-helix. Ligand binding enables the former to “steal” the helix from the latter. (H) Forced circular permutation. Circularly permuting the protein with a short linker forces its original termini together, unfolding it or distorting the binding site. Chemical or enzymatic cleavage eliminates the conformational strain and restores binding.
