Figure 3.
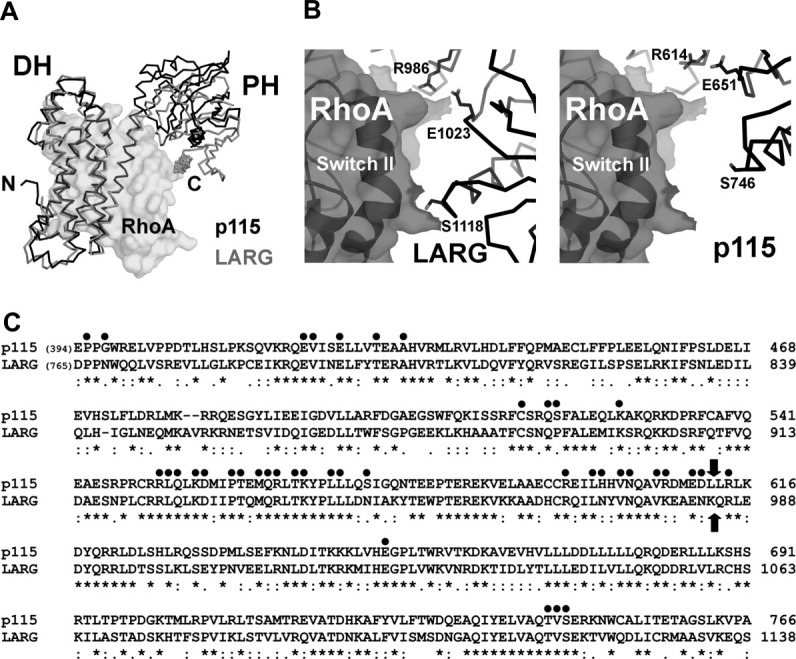
(a) Structural comparison of the DH/PH domains from p115 (black lines) and LARG (gray lines, PDB access code 1X86, with the bound nucleotide-free RhoA depicted as transparent solvent accessible surface). The structural alignment was based on coordinates from the DH domains only. The PH domain in LARG moves closer toward its DH domain upon binding to RhoA. (b) The interface between the PH domain and RhoA. In the crystal structure of the LARG-DH/PH:RhoA complex (left), residues from PH (depicted as sticks) make direct contacts with RhoA (depicted as transparent solvent accessible surface with black ribbons underneath). In the modeled p115-DH/PH:RhoA complex (right), where the two DH domains from p115 and LARG are superimposed, the same set of residues in p115 PH domain can not form direct contacts with RhoA. (c) Sequence alignment of DH/PH domains from p115 and LARG. Residues in LARG that are involved in contacts with the nucleotide-free RhoA are marked with dots on top. Block arrows mark the domain boundary between DH and PH. The sequence alignment is carried out by the program Clustal W.23
