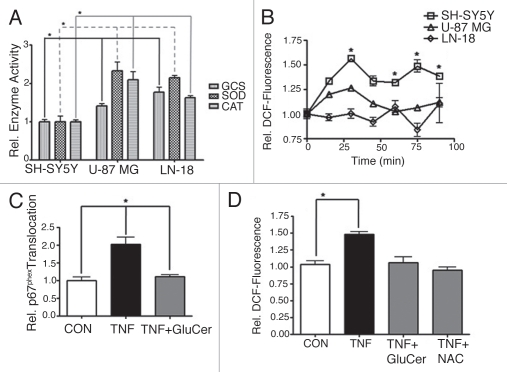
Figure 2.
Glucosylceramide blocks agonist-stimulated NOX activity. (A) Basal activities of catalase (CAT), superoxide dismutase (SOD) and glucosylceramide synthase (GCS) were compared between human SH-SY5Y neuroblastoma, U-87 MG glioblastoma and LN-18 glioblastoma cells. Activities were normalized to the average activities of SH-SY5Y cells. Data represent the mean ± SEM of at least three independent experiments; *p < 0.05, as determined by 1-way ANOVA. (B) The redox-sensitive indicator H2DCFDA was used to compare production of intracellular ROS between SH-SY5Y, U-87 MG or LN-18 cells in response to doxorubicin (8.6 µM). Fluorescence, corresponding to ROS, was normalized to the average 0-min fluorescence of the respective cell line (relative DCF-fluorescence). Data represent the mean ± SEM of three independent experiments; *p < 0.05, as determined by 2-way ANOVA, comparing SH-SY5Y response to both U-87 MG and LN-18 response. (C) Translocation of p67phox to the plasma membrane was evaluated as an indication of NOX assembly. SH-SY5Y cells were exposed to TNFα (TNF, 100 ng/ml, 15 min) ±60 min pretreatment with exogenous C8-glucosylceramide (GluCer, 10 µM). Translocation was normalized to the average translocation of the control. Data represent the mean ± SEM of three independent experiments; *p < 0.01, as determined by 1-way ANOVA. (D) Intracellular ROS production was evaluated in SH-SY5Y cells exposed to TNFα (TNF, 100 ng/ml, 60 min) ±60 min pretreatment with GluCer (10 µM) or the antioxidant N-acetyl-L-cysteine (NAC, 5 mM). Data represent the mean ± SEM of three independent experiments; *p < 0.01, as determined by 1-way ANOVA.