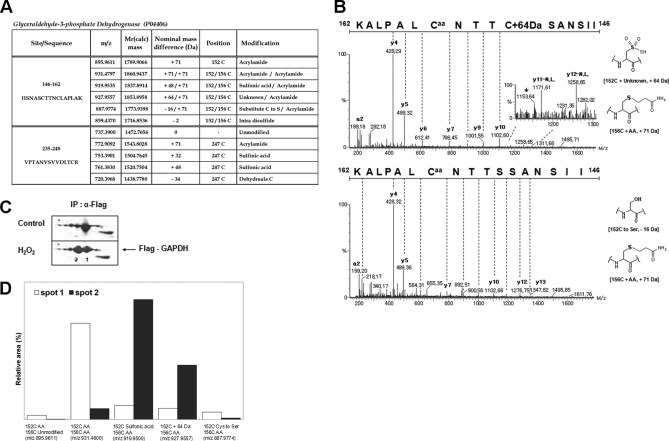
Fig. 1.
Novel modifications of active site cysteine detected in GAPDH. A, Summary of cysteine modifications observed in cellular GAPDH purified from immuno-precipitation of HEK293 cells, by tandem MS. Peptide m/z and calculated mass, and detected nominal mass changes at 152C/156C and 247C residues were presented. B, MS/MS spectra of active site “CXXXC” containing unknown mass shift +64 and −16 Da. Caa indicates acrylamide adduct of Cys residue and star indicates fragment ion that lost H2O or NH3. Presumed chemical structure of each mass shift at Cys residue was presented on the right side. C, The immuno-precipitated GAPDH was separated on 2D-PAGE and detected with Coomassie staining. D, Quantitative analyses of modifications in peptide 146IISNASCTTNCLAPLAK162 based on precursor ion intensities in supplemental Fig. 1B using Glu-fibrinopeptide as an internal standard.