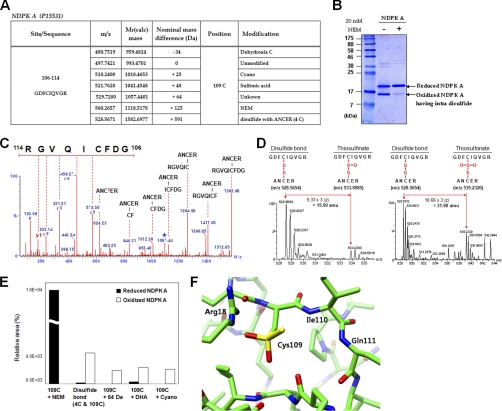
Fig. 2.
The origins of novel Cys modifications analyzed using recombinant NDPK A. A, Summary of observed modifications at 109C in peptide 106GDFCIQVGR114 of purified recombinant NDPK A by MS/MS analysis. Peptide m/z and calculated mass, and detected nominal mass changes at 109C residues were presented. B, Samples analyzed in MS/MS analysis were separated on SDS-PAGE under nonreducing conditions and stained with Coomassie blue. This gel shows that NDPK A contains intradisulfide bonds. C, MS/MS spectrum of intradisulfide bond in a solution of recombinant NDPK A between 106GDFCIQVGR114 and 2ANCER6. Cs indicates cysteine persulfide fragment ion and star indicates fragment ion that lost H2O or NH3. D, MS spectra of thiosulfinate and thiosulfonate through further oxidation of disulfide bond in a solution of recombinant NDPK A. E, Quantitative MS analyses of modifications in peptide 106GDFCIQVGR114 of control and oxidized NDPK A in solution were carried out based on precursor ion intensities in supplemental Fig. 3B using Glu-fibrinopeptide as an internal standard. F, A new modification of Cys109 in the modeling structure of human NDPK A. NDPK A is a homohexameric protein. Each subunit was represented with different colors. The image shows a region around Cys109 located on the chain A of the NDPK A structure. The residues consisting of near Cys109 are represented by stick models. Yellow color represents sulfur, blue for nitrogen, red for oxygen, and green for carbon. The modified Cys109 is stabilized by positively charged environment mainly contributed by Arg18 and partly by two backbone nitrogen atoms of Ile110 and Gln111. There is a room to accommodate the extra sulfur and oxygen of modified Cys109 following energy minimization.