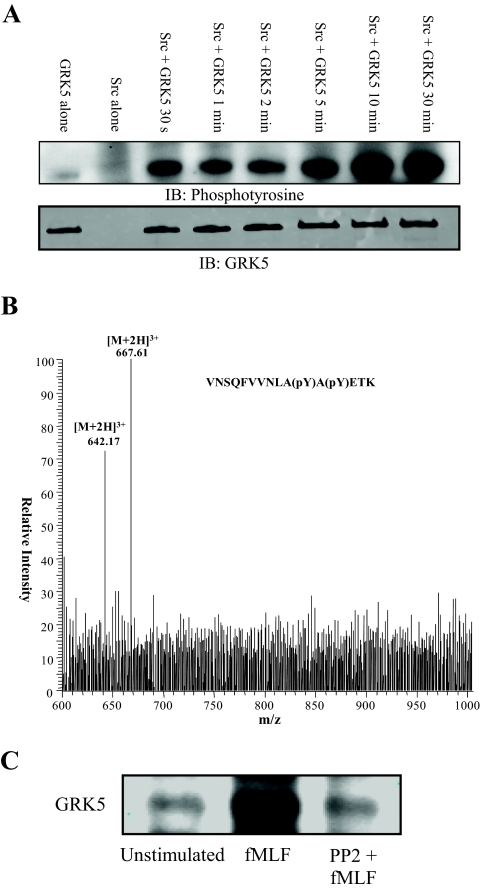
Fig. 3.
Src phosphorylates GRK5. A, Active Src (30 ng) was incubated with or without GRK5 (200 ng) at 30 °C for various times. Proteins were subjected to SDS-PAGE, transferred to PVDF, and analyzed for tyrosine phosphorylation by immunoblot analysis. The blot demonstrates that rapid phosphorylation of GRK5 by Src occurred within 30 s and maximal phosphorylation occurred by 10 min. Parallel blots were probed with anti-GRK5 to confirm similar amounts of GRK5 in each reaction. B, To confirm that Src phosphorylates Tyr 251 and Tyr 253 of GRK5, an in-solution trypsin/Lys-C digest of an in vitro kinase reaction was subjected to mass spectrometry. Precursor ion scanning for product ions yielding the phosphotyrosine-specific immonium ion (216.04 m/z) identified precursor ion masses of 642.17 m/z and 667.61 m/z. These masses correspond to the [M+2H]3+ singly- and doubly phosphorylated GRK5 peptide VNSQFVVNLApYApYETK, where pY represents phosphorylation of tyrosine. C, Neutrophils were pretreated with or without 10 μm PP2 at 37 °C for 10 min. Cells were then treated with or without 0.3 μm fMLF for 2 min after which reactions were stopped by resuspension in ice-cold lysis buffer. Proteins were immunoprecipitated from lysates with anti-phosphotyrosine conjugated to agarose. Proteins were separated by SDS-PAGE and subjected to immunoblot analysis for GRK5. An increased amount of GRK5 was precipitated from cells stimulated with fMLF, whereas pretreatment with the Src inhibitor PP2 returned the amount of precipitated GRK5 to basal levels.