Abstract
Purpose
To evaluate the effects of timing and length of zoledronic acid (ZA) treatment on outcomes for patients with prostate cancer in clinical practice.
Materials and methods
Patients with prostate cancer and first bone metastasis diagnosed from January 2003 to October 2006 were included. Patients were considered ‘untreated’ if no ZA was given, ‘early ZA-treated’ if ZA was initiated before skeletal complication (SC) occurrence or ‘late ZA-treated’ if one or more SC was documented before or at ZA initiation. Patients were classified with short (≤90 days), medium (91–180 days) or long (>180 days) treatment persistence. Assessments included follow-up duration (FUP) and risk of developing one or more SC.
Results
Among eligible patients, 847 were untreated, 243 were early ZA-treated and 218 were late ZA-treated. For untreated versus early ZA-treated groups, median FUP was 263 versus 357 days (p<0.0001), respectively, and time to first SC was 199 versus 273 days (p<0.0001), respectively. ZA treatment was associated with significantly longer FUP and lower SC risk. The early ZA-treated group had significantly longer FUP versus the late ZA-treated group (median days, 357 vs. 299.5); the late ZA-treated group experienced significantly higher SC risk vs. the early ZA-treated group (odds ratio, 1.51). Compared with the long-persistence group, FUP was 56% and 40% shorter in the short and medium groups, respectively (p<0.0001).
Conclusion
Treatment with and early initiation of ZA for patients with prostate cancer and bone metastasis significantly prolonged time to and reduced risk of developing SC, while extending FUP.
Keywords: Bone metastasis, Prostate, Prostatic neoplasms, Skeletal complication, Zoledronic acid
Introduction
Prostate cancer is the second most common cancer diagnosed in men world-wide 1. It is characterized by the propensity for developing bone metastases (BMs), which occur in more than 80% of patients with advanced disease2. Patients with prostate cancer are at high risk for skeletal complications (SCs) from BM and bone loss induced by cancer treatments (e.g., androgen-deprivation therapy)1,3.
With a median survival of 30–35 months after a BM diagnosis, patients with prostate cancer experience considerable morbidity, adversely affecting patients’ health-related quality of life and substantially increasing medical costs4. One study reported significantly higher total treatment costs (excluding bisphosphonate therapy) for those patients experiencing one or more SC ($7522) compared with those not experiencing an SC ($4180) in year 2000 US dollars5. In a recent retrospective study, the annual economic impact of treating SC in patients with prostate cancer with BM in the year after initial SC diagnosis was $12 4696.
A review of several prostate cancer studies reported that bisphosphonates reduced SC and bone pain and reduced the adverse effects of androgen-deprivation therapy7. Zoledronic acid (ZA) is approved for treatment of BM in patients with prostate cancer who have progressed after one or more hormonal therapy. ZA decreased SC in a randomized controlled trial of patients with hormone-refractory metastatic prostate cancer8,9. ZA significantly decreases the incidence, delays onset and reduces overall risk of SC compared with placebo and has a well-recognized safety profile10. Controlled clinical trials also have demonstrated the long-term efficacy of ZA in preventing or reducing the risk of SC in men with advanced prostate cancer and BMs11,12. In elderly patients with BMs from solid tumors, the benefits of ZA in reducing pain and increasing health-related quality of life were reported13. Moreover, a review of in vitro and in vivo studies on the antitumor activity of bisphosphonates suggests that ZA may stop disease progression and reduce tumor burden in bone14.
Given the central role that ZA plays in managing SC in patients with prostate cancer, a drug use review was conducted using a nationally representative claims database to further quantify the effect of ZA and to address questions regarding when to start ZA therapy and how long to remain on treatment15. The results indicated that greater persistence on ZA was associated with reduced risk of SC and prolonged follow-up duration (FUP) in a cohort of patients with solid tumors having experienced BM and one or more SC15. The present study incorporates an additional year of follow-up data and takes into account the unique aspects of managing patients with prostate cancer as it pertains to the experience of SC within the context of using intravenous (IV) bisphosphonate in clinical practice settings.
Materials and methods
Study design
This retrospective cohort study utilized the PharMetrics integrated managed-care claims database, which is nationally representative of employer-based health plans. Claims of more than 55 million patients from more than 80 United States (US) health plans were compiled. Data from January 2002 to October 2006 were analyzed, and patients with prostate cancer who had their first BM diagnosed from January 2003 to October 2006 were selected from the database. Men with a diagnosis of prostate cancer (International Classification of Diseases, 9th Revision [ICD-9] code 185) and BM (ICD-9 code 198.5: secondary malignant neoplasm of bone and bone marrow) and who had been enrolled in the plan for ≥6 months prior to the initial BM diagnosis were selected. SC claims, including radiation therapy to bone (Current Procedural Terminology, version 4, code 77413), spinal cord compression (ICD-9 code 336.9), pathologic fracture of vertebrae (ICD-9 code 733.13) and bone surgery (ICD-9 code 78.10) also were collected. To avoid counting a continuous SC event more than once, a 21-day window as previously reported in a controlled trial was applied16. The number of SCs was adjusted with FUP to derive a monthly SC rate. The 6-month baseline before BM was used to identify potential baseline comorbidities. Patients who had neoplasms other than prostate cancer or multiple cancers were excluded, as were patients treated with IV bisphosphonates other than ZA.
Three groups were evaluated: (1) untreated patients who did not have a diagnosis of SC before BM and were not treated with ZA or other IV bisphosphonates; (2) early ZA-treated patients who did not have a diagnosis of SC before BM and first ZA treatment; and (3) late ZA-treated patients who had one or more SC documented before or at the initiation of ZA. Based on the US label recommending a ZA dosing schedule of every 3–4 weeks9, treatment persistence was defined as the number of days from ZA treatment initiation to the date of the first gap more than 45 days between consecutive treatments. Patients were categorized as having short (≤90 days), medium (91–180 days) or long (>180 days) treatment persistence.
Outcomes assessment
The impact of ZA treatment patterns on outcomes was evaluated in three steps. First, ZA treatment was compared with no treatment regarding impact on the following patient outcomes: (1) FUP from first BM diagnosis to last claim available; (2) time to first SC; and (3) rate of SC after BM. Analyses then were performed on early ZA-treated versus late ZA-treated to investigate the risk and rate of SC after ZA initiation and the impact of treatment timing on FUP. Finally, the impacts of persistence with ZA on FUP, time to first SC and rate of SC after BM were examined by grouping the early ZA-treated patients according to their degree of persistence.
Statistical analyses
The propensity score method was used to control for possible selection bias in ZA administration17. Age, score on Charlson Comorbidity Index (CCI)18,19, geographic location, year of first BM diagnosis, other metastases, more than one BM claim and opioid or oral bisphosphonate use before first BM diagnosis were entered into a logistic regression model as predictors of ZA treatment. Factors significantly associated with ZA administration were combined to calculate propensity scores, grouped by quintiles and entered as covariates in multiple regression models.
Means, medians and interquartile ranges (IQRs) were reported and appropriate statistical tests were performed. Moreover, depending on the studied outcomes, several types of multiple regression analyses were performed.
Treatment with docetaxel, a first-line agent in patients having castration-resistant prostate cancer, was included to identify patients with hormone-refractory prostate cancer20.
All analyses were conducted using SAS 9.0 statistical software (SAS Institute, Inc, Cary, NC, USA).
Results
Patient characteristics by treatment groups
Among patients who met the inclusion criteria, 847 were untreated, 243 received ZA treatment before the development of an SC (early ZA-treated) and 218 received ZA treatment after or at the same time as occurrence of an SC (late ZA-treated).
Comparisons between untreated and early ZA-treated patients
The untreated group was significantly older and had a higher mean CCI score than the early ZA-treated group (both p<0.0001; Table 1). The proportion of patients treated with docetaxel was significantly higher in the early ZA-treated than the untreated group (46.1 vs. 6.4%, p<0.0001). The median (IQR) FUP from BM to last claim was 263 (461) days in the untreated and 357 (398) days in the early ZA-treated group (p<0.0001). Time to first SC was 199 (394) days for the untreated and 273 (364) days for the early ZA-treated group (p<0.0001). No significant difference in the proportions of patients with one or more SC after BM was observed (22.7% in the untreated group vs. 27.2% in the ZA-treated group). However, when the number of SC was adjusted for the longer FUP experienced by the ZA-treated group, the rate was significantly lower in the early ZA-treated than the untreated group (mean [SD]: 0.02 [0.15] vs. 0.05 [0.16] per month, respectively; p=0.012).
Table 1.
Characteristics of the untreated and ZA-treated groups.
| Treatment group | Patients, n | Age, mean (SD) | CCI score, mean (SD) | Docetaxel treatment, n (%) |
|---|---|---|---|---|
| Untreated | 847 | 68.03 (13.66) | 8.88 (1.30) | 54 (6.38) |
| Early ZA-treated | 243 | 64.51 (11.19) | 8.49 (0.85) | 112 (46.09) |
| Late ZA-treated | 218 | 66.19 (11.63) | 8.60 (0.91) | 103 (47.25) |
| Short ZA persistence | 87 | 66.74 (12.47) | 8.63 (1.11) | 34 (39.08) |
| Medium ZA persistence | 58 | 63.78 (11.49) | 8.47 (0.71) | 25 (43.10) |
| Long ZA persistence | 98 | 62.96 (9.48) | 8.37 (0.63) | 53 (54.08) |
SD, standard deviation; ZA, zoledronic acid.
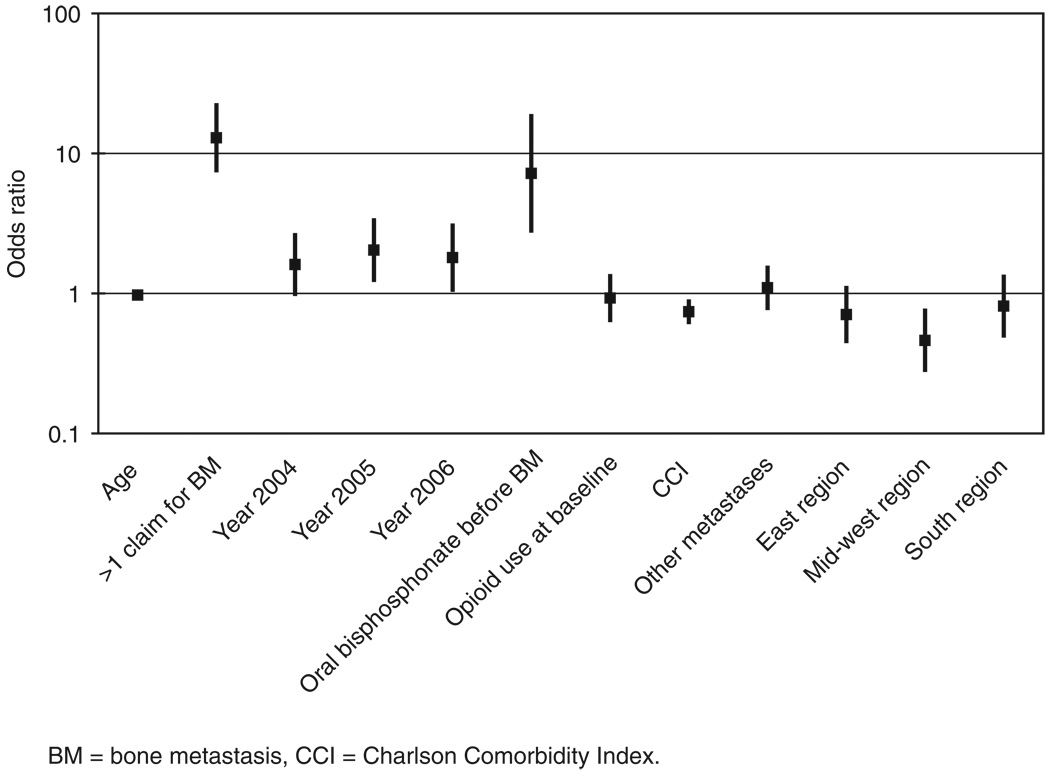
Propensity score calculations revealed that patients more likely to receive early ZA therapy were those diagnosed with BM in more recent years, who used an oral bisphosphonate before BM, who had other metastases before BM, who had more than one claim for BM, who resided in the West region (compared with those in the Midwest), who were younger and who had lower CCI scores (all p<0.05; Figure 1).
Figure 1.
Odds ratios and 95% confidence interval estimated from logistic regression on the propensity to receive early ZA treatment compared with no treatment (i.e., untreated). Comparison group was younger and had only one BM claim, BM diagnosed in 2003, no use of oral bisphosphonate before BM, no use of opioids at baseline, lower CCI score, no other metastases and residence in the West region. Significant variables were younger age, more than one claim before BM, BM diagnosed in 2005 or 2006, use of oral bisphosphonate before BM, lower CCI score and residence in the Mid-west region.
After adjustment with regression models, it was found that ZA significantly increased FUP (p<0.05), while older age and higher CCI scores reduced FUP (p<0.0001). Docetaxel-treated patients also experienced significantly longer FUP (p<0.05). On average, the early ZA-treated group had 28.5% (95% CI: 4.2%, 58.6%) longer FUP compared with the untreated group. Regression modeling also found that early ZA treatment significantly reduced the risk of SC compared with the untreated group (hazard ratio: 0.463; 95% CI 0.280, 0.679; p<0.001), while age, CCI score and docetaxel-treatment status were not significant. Moreover, there was a significant trend for SC risk to increase over time (p<0.05). Regression model on SC incidence showed that early ZA use reduced the number of SC by 33% (95% CI 1.2%, 54.5%; p<0.05) compared with the untreated group, while age and CCI score increased SC incidence (p<0.05; Table 2).
Table 2.
Results of regression models comparing untreated and early ZA-treated groups.
| Log of FUP: coefficient (SE) from multiple regression |
Time to first SC: HR (95% CI) from Cox regression |
No. of SCs: coefficient (SE) from Poisson regression |
|
|---|---|---|---|
| Age | −0.014 (0.003)* | 1.008 (0.997–1.019) | 0.016 (0.006)† |
| CCI score | −0.143 (0.033)* | 1.097 (0.974–1.235) | 0.134 (0.066)† |
| Docetaxel treatment | 0.281 (0.118)† | 1.291 (0.911–1.830) | 0.116 (0.207) |
| Early ZA treatment | 0.251 (0.107)†a | 0.463 (0.280–0.679)‡ | −0.399 (0.198)†b |
| Time × early ZA treatmentc | Not included | 1.002 (1.001–1.003)† | Not included |
p<0.0001;
p<0.05;
p<0.001.
Because the dependent variable is log-transformed follow-up duration, the exponential of this regression coefficient represents ratio of follow-up duration of the late ZA-treated group to that of the early ZA-treated group. The exponential of 0.251 is 1.285. Therefore, the early ZA-treated group had a follow-up duration about 28.5% longer than the untreated group.
In Poisson regression, the exponential of this regression coefficient represents the ratio of skeletal complications in the late ZA-treated group to those in the early ZA-treated group. The exponential of −0.399 is 0.671; therefore, the early ZA-treated group had about 33% fewer skeletal complications than the untreated group, after controlling for follow-up duration.
The interaction term between time and early ZA treatment was included only in the Cox regression model.
CCI, Charlson Comorbidity Index; CI, confidence interval; FUP, follow-up duration; HR, hazard ratio; SC, skeletal complications; SE, standard error; ZA, zoledronic acid.
Comparisons between early and late ZA-treated groups
Early and late ZA-treated groups were comparable in age and CCI scores (p>0.05, Table 1). Neither geographic location nor year of first BM diagnosis influenced the timing of ZA treatment initiation. The two groups had similar proportions of patients who were docetaxel-treated (46.1% in early-treated groups and 47.3% in late-treated, p>0.05). In the unadjusted analyses, the early ZA-treated group had significantly longer FUP days than the late ZA-treated group (median [IQR] days, 357 [398] vs. 299.5 [384], respectively, p<0.05). In addition, there was a smaller proportion of early ZA-treated patients (27.2%) than late ZA-treated patients who developed a SC (35.8%; p<0.05) and had a significantly lower monthly rate of SC after early ZA treatment compared with late ZA treatment (mean [SD]: 0.03 [0.07] vs. 0.08 [0.21], respectively; p<0.005).
After adjusting for age, CCI score and docetaxel treatment, the late ZA-treated group had 17.5% (95% CI: 2.9%, 30.0%; p<0.05) shorter FUP than the early ZA-treated group. Additionally, higher CCI score was associated with reduced FUP, and docetaxel treatment was associated with increased FUP (both p<0.001). The risk of SC after initiation of ZA was 50% higher in the late ZA-treated group than in the early ZA-treated group (odds ratio [OR]: 1.508, p<0.005, Table 3). Docetaxel treatment was associated with a significantly higher risk of having one or more SC after ZA treatment (OR: 1.658, p<0.05). Regression modeling also showed that the SC incidence was significantly higher in the late ZA-treated group, who experienced 57.5% (95% CI: 9.3%, 126.8%; p<0.05) more SC than the early ZA-treated group.
Table 3.
Results of regression models comparing early and late ZA-treated patients.
| Log of FUP: coefficient (SE) from multiple regression |
Risk of SC after ZA: OR (95% CI) from logistic regression |
No. of SCs: coefficient (SE) from Poisson regression |
|
|---|---|---|---|
| Age | −0.004 (0.004) | 1.001 (0.982–1.020) | <0.001 (0.009) |
| CCI score | −0.143 (0.049)* | 0.901 (0.704–1.155) | 0.012 (0.118) |
| Docetaxel | 0.340 (0.076)* | 1.658 (1.106–2.485)† | 0.025 (0.191) |
| Late ZA-treated | −0.193 (0.083)† | 1.508 (1.010–2.251)† | 0.454 (0.186)† |
p<0.001;
p<0.05.
CCI, Charlson Comorbidity Index; CI, confidence interval; FUP, follow-up duration; OR, odds ratio; SC, skeletal complications; SE, standard error; ZA, zoledronic acid.
Comparisons among outcomes of ZA treatment by persistence groups
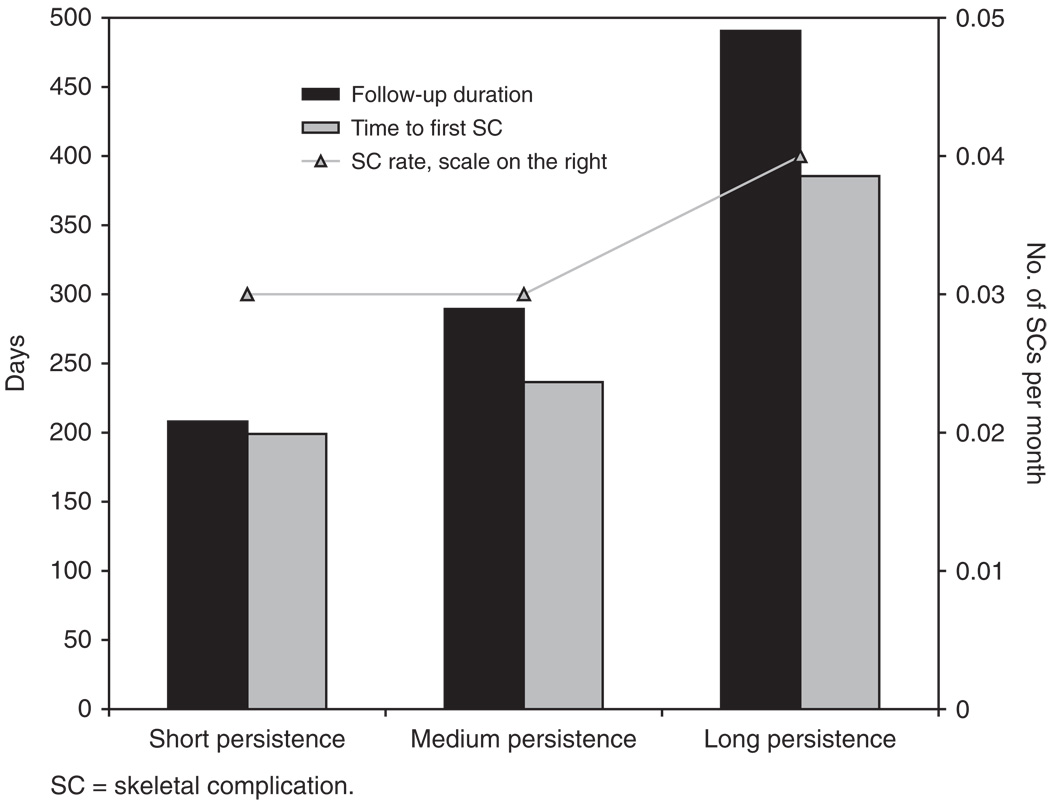
The mean age, CCI score and docetaxel-treatment status of the 243 early ZA-treated patients evaluated by level of treatment persistence were generally comparable. Although the long-persistence group had the greatest proportion of patients who were docetaxel treated compared with the other persistence groups, the chi-square test result was not significant. Unadjusted outcomes showed statistically significant differences in proportions of patients with one or more SC (short persistence, 21%; medium persistence, 17%; long persistence, 39%), median FUP and median time to first SC after BM (all p<0.05), but not the mean monthly SC rate (p>0.05, Figure 2).
Figure 2.
Outcomes of early ZA-treated patients by level of treatment persistence. There was a significant difference among the three groups in median time of FUP and median time to first SC (p<0.05) but not in mean monthly SC rate.
Using the long-persistence group as a reference, short and medium treatment persistence were associated with significantly shorter FUP after multivariate adjustment (p<0.0001; Table 4). Compared with the long-persistence group, FUP was 56% and 40% shorter in the short and medium groups, respectively. No significant differences were observed between the persistence groups in risk or rate of SC (p>0.05). Docetaxel treatment was associated with longer FUP (p<0.0001) but did not have a significant impact on either the risk or rate of SC (p>0.05).
Table 4.
Results of regression models comparing the three ZA-persistence groups.
| Log of FUP: coefficient (SE) from multiple regression |
Time to first SC: HR (95% CI) from Cox regression |
No. of SCs: coefficient (SE) from Poisson regression |
|
|---|---|---|---|
| Age | 0.002 (0.005) | 0.988 (0.963–1.013) | −0.004 (0.014) |
| CCI score | −0.092 (0.060) | 1.157 (0.843–1.589) | 0.092 (0.173) |
| Docetaxel treatment | 0.421 (0.100)* | 1.458 (0.881–2.412) | 0.147 (0.270) |
| Short ZA persistence | −0.819 (0.114)* | 0.683 (0.382–1.222) | −0.280 (0.315) |
| Medium ZA persistence | −0.510 (0.126)* | 0.551 (0.273–1.112) | −0.284 (0.342) |
p<0.0001.
CCI, Charlson Comorbidity Index; CI, confidence interval; FUP, follow-up duration; HR, hazard ratio; SC, skeletal complications; SE, standard error; ZA, zoledronic acid.
Discussion
The present study evaluated the managing patterns in patients with prostate cancer and BM as it relates to the use of the IV bisphosphonate, ZA, in the clinical setting. The study found that ZA use in this patient population is associated with significantly reduced SC risk compared with no treatment and significantly prolonged time to SC onset. More important, the timing of ZA treatment initiation is associated with positive clinical outcomes, with the best outcomes achieved in patients initiating treatment earlier in the course of metastatic disease and persisting on treatment longer without gaps of more than 45 days between consecutive administrations. The degree of therapy persistence also may influence patient outcomes. The present study shows that persistence on ZA treatment at a frequency of every 3–4 weeks is associated with a patient’s FUP, which are nearly 56% shorter in the short-persistence group and 40% shorter in the medium-persistence groups, compared with the long-persistence group. No significant differences in the risk or rates of SC were observed between-persistence groups.
The study findings are relevant in the context of the clinical picture for patients with prostate cancer who develop BM and in light of earlier reported studies11,12. With the high prevalence for BM in these patients, health-care professionals are faced with multiple challenges in complication management. Malignant bone lesions affect skeletal structural integrity, resulting in SC (i.e., pathologic fracture, spinal cord compression and severe bone pain), all of which adversely affect patients’ health-related quality of life21. For this reason, maintaining skeletal integrity and preventing SC are essential to the well-being of patients with prostate cancer. Consequently, early diagnosis and treatment of bone loss and BMs with bisphosphonates are recommended1. It also is recommended that bisphosphonate therapy be sustained throughout disease duration22.
In an analysis of three large, randomized controlled trials, ZA was associated with a significant reduction in the cumulative SC incidence compared with placebo in patients with prostate cancer23. Long-term ZA treatment also afforded continuing clinical benefits in patients with advanced prostate cancer, even after the occurrence of SC11. Recent data suggest that early treatment with ZA before the onset of bone pain may be associated with anti-tumor effects and may positively affect survival in subsets of patients with elevated levels of N-telopeptide of type I collagen, a biochemical marker of bone resorption24. In the majority of patients with prostate cancer or other solid tumors who received ZA, normalization of elevated N-telopeptide of type I collagen levels was observed and correlated with extended survival24.
In the present study, the significantly increased FUP associated with ZA treatment, especially in patients who persisted longer on ZA therapy, is notable when considering preliminary evidence suggesting both antitumor/antimetastatic properties and improved patient survival associated with ZA. The antitumor/antimetastatic properties include the inhibition of angiogenesis, tumor cell invasion and bone adhesion and antitumor synergy with cytotoxic chemotherapy25,26. Additionally, one recent study found that adding ZA to adjuvant endocrine therapy improved disease-free survival in premenopausal women with estrogen-responsive early breast cancer, while another showed that adding ZA to a combination chemotherapy regimen increased overall survival in patients with advanced (stage IV) lung cancer and BMs25,26. The latter study also showed that longer ZA persistence correlated with longer survival and time to progression26.
In addition to the long-term data establishing ZA efficacy and safety in patients with prostate cancer from clinical trials11,12, the seemingly positive impact of treatment with ZA in the clinical setting has been previously reported15. A recent retrospective claims study, which was used as a foundation for the current study, reported an association between ZA treatment and both the reduction in the skeletal morbidity rate and the delay in time to occurrence of SC in a combined cohort of patients with a diagnosis of BM and a solid tumor who experienced one or more SC15. Greater persistency of ZA use was also associated with lower monthly rates of SC15.
The present study has several limitations. The PharMetrics database has a potential bias toward employer-type managed health plans, which may limit the generalizability of the results to other populations. Being an observational study, the selection bias might not be fully accounted for even with the use of the propensity score method. The severity of the patients’ clinical status, BM and SC and the disposition of patients after disenrollment could not be fully ascertained in the claims data. Despite these limitations, the use of a claims database permitted the examination of a larger patient sample than would be possible using a chart review-based study.
Conclusions
The results of this study suggest that ZA therapy in patients with prostate cancer is associated significantly and positively with prolonged time to and reduced risk for developing SC, while extending FUP. Best outcomes were observed in patients who initiated ZA treatment earlier in the course of metastatic disease and persisted on indicated dosing frequency (i.e., no gaps of >45 days between two consecutive administrations). These findings may argue for earlier intervention and longer persistence with ZA in patients with prostate cancer with BMs.
Acknowledgments
The editorial assistance of ApotheCom in finalizing this manuscript is acknowledged.
Transparency
Declaration of funding
The study was supported by Novartis Pharmaceuticals.
Declaration of financial/other relationships
H.T.H. has disclosed that she is a paid consultant to Novartis, the marketer of zoledronic acid. S.-J.L. has disclosed that she is a consultant to Hind T. Hatoum & Company. M.R.S. and A.L. have disclosed that they act in an advisory capacity with Novartis. In addition, A.L. has disclosed that he has received laboratory research funding and provided expert testimony for Novartis. A.G. has disclosed that she is an employee of Novartis. The authors had full control of the findings and results presented, without any oversight or interference from the sponsor of the work.
The study was conducted using a licensed data set from PharMetrics to Novartis. PharMetrics data sets are structured to maintain patient anonymity and are in compliance with the Health Insurance Portability and Accountability Act (HIPAA). PharMetrics data sets do not contain patients’ names; rather, patients are given unique identifying numbers to enable the conduct of research at patient level such as that reported in this manuscript.
Footnotes
Parts of these data were previously presented at the IX International Meeting on Cancer Induced Bone Disease, October 28–31, 2009, in Arlington, Virginia, USA.
Contributor Information
Hind T. Hatoum, University of Illinois at Chicago and Hind T. Hatoum & Company, Chicago, IL, USA
Swu-Jane Lin, University of Illinois at Chicago and Hind T. Hatoum & Company, Chicago, IL, USA.
Amy Guo, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Allan Lipton, Milton S. Hershey Medical Center, Hershey, PA, USA.
Matthew R. Smith, Massachusetts General Hospital, Boston, MA, USA
References
- 1.Saad F, Sternberg CN. Multidisciplinary management of bone complications in prostate cancer and optimizing outcomes of bisphosphonate therapy. Nat Clin Pract Urol. 2007;4:S3–S13. doi: 10.1038/ncpuro0727. [DOI] [PubMed] [Google Scholar]
- 2.Yuen KK, Shelley M, Sze WM, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD006250. CD006250. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Chi K, Fleshner N. NThe role of bisphosphonates in the management of bone metastases in prostate cancer. Can J Urol. 2004;11:2376–2382. [PubMed] [Google Scholar]
- 4.Groot MT, Boeken Kruger CGG, Pelger RCM, et al. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol. 2003;43:226–232. doi: 10.1016/s0302-2838(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 5.Reed SD, Radeva JI, Glendenning GA, et al. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol. 2004;171:1537–1542. doi: 10.1097/01.ju.0000116777.94426.60. [DOI] [PubMed] [Google Scholar]
- 6.Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14:317–322. [PubMed] [Google Scholar]
- 7.Guise TA, Chirgwin JM. Role of bisphosphonates in prostate cancer bone metastases. Semin Oncol. 2003;30:717–723. doi: 10.1016/s0093-7754(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 8.Lattouf JB, Saad F. Preservation of bone health in prostate cancer. Curr Opin Support Palliat Care. 2007;1:192–197. doi: 10.1097/SPC.0b013e3282f0c74f. [DOI] [PubMed] [Google Scholar]
- 9.Novartis: Zometa® (zoledronic acid) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2008. [Google Scholar]
- 10.Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4:261–268. doi: 10.2147/tcrm.s2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad F, Chen YM, Gleason DM, et al. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 12.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 13.Addeo R, Nocera V, Faiola V, et al. Management of pain in elderly patients receiving infusion of zoledronic acid for bone metastasis: a single-institution report. Support Care Cancer. 2008;16:209–214. doi: 10.1007/s00520-007-0315-y. [DOI] [PubMed] [Google Scholar]
- 14.Hoesl CE, Altwein JE. Biphosphonates in advanced prostate and renal cell cancer—current status and potential applications. Urol Int. 2006;76:97–105. doi: 10.1159/000090869. [DOI] [PubMed] [Google Scholar]
- 15.Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer. 2008;113:1438–1445. doi: 10.1002/cncr.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 17.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Dreicer R. Current status of cytotoxic chemotherapy in patients with meta-static prostate cancer. Urol Oncol. 2008;26:426–429. doi: 10.1016/j.urolonc.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Saad F, Clarke N, Colombel M. Natural history and treatment of bone complications in prostate cancer. Eur Urol. 2006;49:429–440. doi: 10.1016/j.eururo.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Aapro MS. Management of bisphosphonate treatment in clinical practice. Semin Oncol. 2007;34:S28–S32. doi: 10.1053/j.seminoncol.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Major PP, Cook RJ, Chen BL, et al. Survival-adjusted multiple-event analysis for the evaluation of treatment effects of zoledronic acid in patients with bone metastases from solid tumors. Support Cancer Ther. 2005;2:234–240. doi: 10.3816/SCT.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Lipton A. Clinical benefits and considerations of bisphosphonate treatment in metastatic bone disease. Semin Oncol. 2007;34:S17–S23. doi: 10.1053/j.seminoncol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 26.Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705–1709. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]