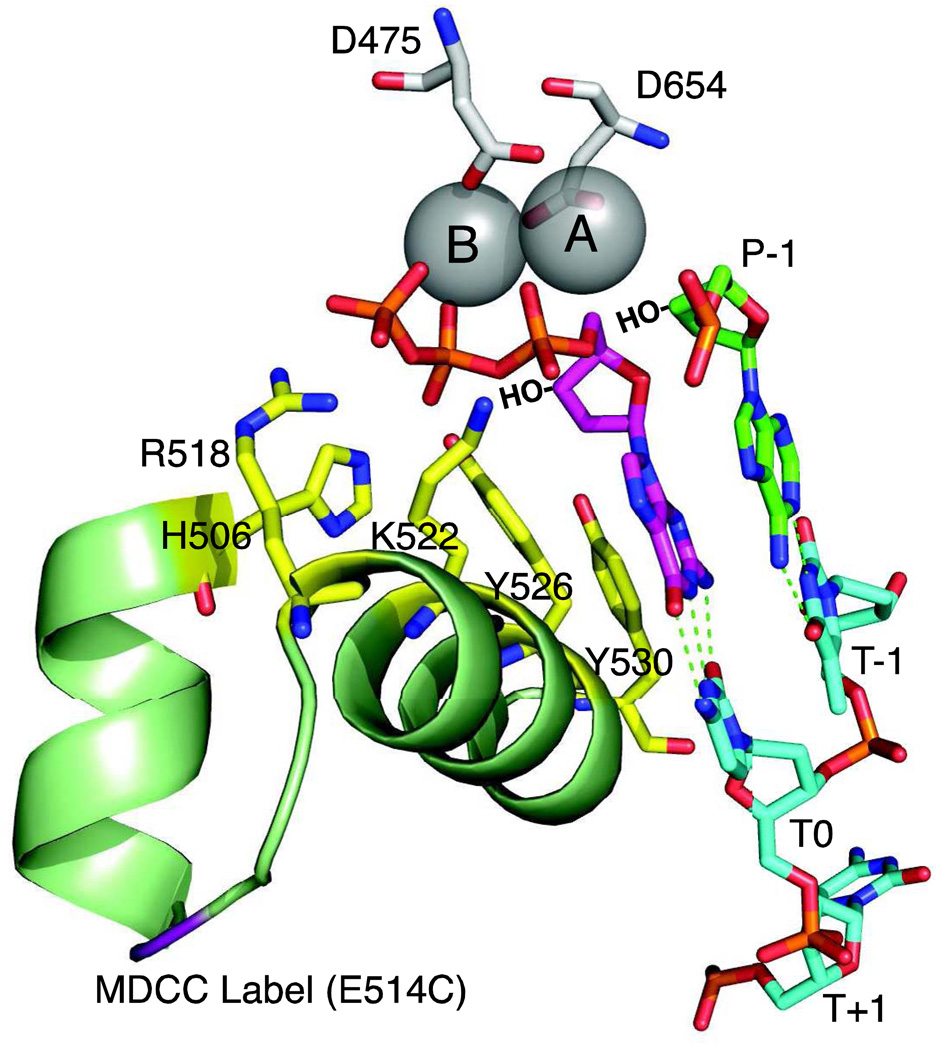
Figure 4. Polymerase active site residues.
The active site of T7 DNA polymerase is shown derived from 1T7P.pdb [1]. Aspartate residues D475 and D654 hold two metal ions (A and B) in place. Important catalytic residues from the fingers domain, (R518, H506, K522, Y526 and Y530, shown in yellow) make contact only in the closed state. The incoming dGTP is shown in magenta, the primer is in green and the template in cyan. Template positions are labeled T−1 through T+1. The 3'OH groups, lacking in the crystal structure, are shown by HO−. The site of the MDCC label is shown in purple.