Table 2.
Activity of R3-substituted compounds against TbPTR1, TbDHFR and human DHFR, and T. brucei and MRC5 cells. R1 in all compounds is 3,4-dichlorobenzyl
| Compd | R3 |
TbPTR1 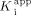 [μm] [μm] |
HsDHFR IC50 [μm] | TbDHFR IC50 [μm] | T. brucei[a] EC50 [μm] | MRC5 EC50 [μm] |
|---|---|---|---|---|---|---|
| 11[b] | H | 0.8 | >50 | >50 | 11 | 27 |
| 24 | 4-Cl | 2.3 | >50 | >30 | ND[d] | ND |
| 25 | 4-O(CH2)2CH3 | 0.31 | >50 | >30 | ND | ND |
| 26 | 4-OBn | 16 | >50 | >50 | >30 | >30 |
| 27 | 4-Ph | >60 | >30 | >30 | ND | ND |
| 28[c] | 4-OMe | 0.46 | >50 | >30 | ND | ND |
| 29[b] | 7-Cl | 0.51 | >50 | >30 | ND | ND |
| 30[b] | 7-O(CH2)2CH3 | 0.047 | >50 | >30 | 9.6 | 21 |
| 31 | 7-OBn | 0.098 | >50 | >50 | 6.7 | 17 |
| 32[b] | 7-Ph | 0.007 | >50 | >50 | 9.9 | >30 |
| 33 | 7-OMe | 0.65 | >50 | >30 | ND | ND |
| 34 | 5,6-dimethyl | 3.9 | >30 | >30 | ND | ND |
