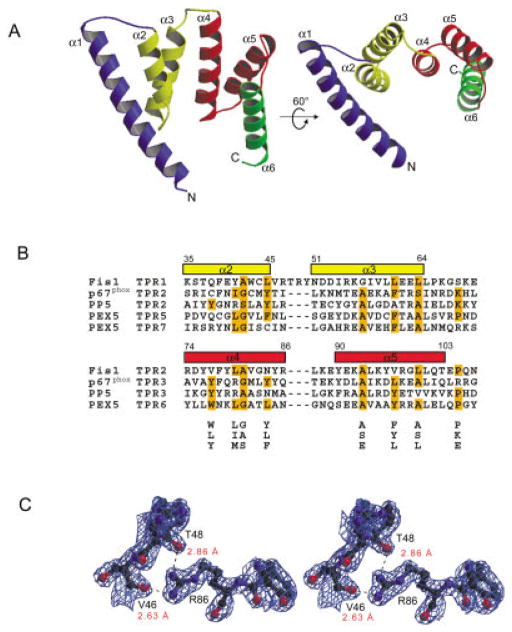
Fig. 1.
TPR fold of Fis1. A: Two ribbon drawings of Fis1 with labeled helices (α1–α6) and TPR motifs color coded as follows: helix α1 blue, TPR1 (α2–α3) yellow, TPR2 (α4–α5) red and helix α6 green. Electron density was not observed for the first two and last two residues. Figures were prepared with MOLSCRIPT23 and rendered in Raster3d.24 B: Structure-based alignment of TPR motifs. The TPR motifs of p67phox, PP5, and PEX5, structural homologs of Fis1 as found by DALI, VAST, and CE, are aligned with the TPR motifs of Fis1. Residues that conform to the TPR consensus sequence below the alignment are highlighted in orange.25 Fis1 α-helical regions are indicated above the alignment by rectangles color-coded as in Figure 1(A). Helix α6 lacks the 34-residue TPR motif and is, therefore, excluded from the alignment. C: Stereoview of the final σA weighted 2Fo −Fc electron density map displaying interactions between labeled residues on helices α2 and α4 calculated to 2.0 Å resolution and contoured at 1.1α. Hydrogen bonds are indicated by dashes, and distances are labeled in red. The figure was generated using BOBSCRIPT26 and rendered in Raster3d.24